Chapter 2: Chemical Basis of Life
... at the basic principles of chemistry as they apply to life processes. In fact, it is almost impossible to speak of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactio ...
... at the basic principles of chemistry as they apply to life processes. In fact, it is almost impossible to speak of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactio ...
Topic 4: Classifying Elements What did the early chemists use to
... subscript can also be used to indicate the state that the element/compound is found in at room temperature. EXAMPLE: H2O(l), NaCl(s) What is a SUPERSCRIPT? What does it indicate? Pro ...
... subscript can also be used to indicate the state that the element/compound is found in at room temperature. EXAMPLE: H2O(l), NaCl(s) What is a SUPERSCRIPT? What does it indicate? Pro ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
Day 5 Intro-to-Chem
... Sand (silicon dioxide or SiO2) Rubbing Alcohol (isopropanol or C3H7OH) Carbon dioxide (CO2) Ammonia (NH3) Methane (CH4) ...
... Sand (silicon dioxide or SiO2) Rubbing Alcohol (isopropanol or C3H7OH) Carbon dioxide (CO2) Ammonia (NH3) Methane (CH4) ...
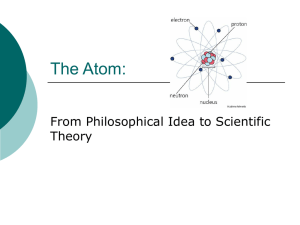
The Atom - Williamstown Independent Schools
... Atoms cannot be subdivided, created or destroyed Atoms of different elements combine in simple, whole number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
... Atoms cannot be subdivided, created or destroyed Atoms of different elements combine in simple, whole number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
Storage Pattern for Chemicals Where Space is Limited
... Storage cabinets for acids, bases and flammables are meant for liquids, not dry solids. Vent acid cabinets to prevent vapor build-up. Store concentrated sulfuric acid on one shelf of the acid cabinet and concentrated hydrochloric acid on another. Store nitric acid in a secondary container with other ...
... Storage cabinets for acids, bases and flammables are meant for liquids, not dry solids. Vent acid cabinets to prevent vapor build-up. Store concentrated sulfuric acid on one shelf of the acid cabinet and concentrated hydrochloric acid on another. Store nitric acid in a secondary container with other ...
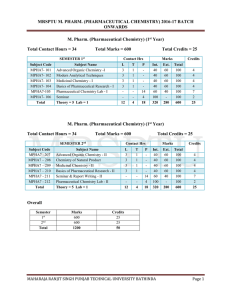
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... 2. Kinetic Studies: First Order Reactions, Second Order Reactions, Third Order Reactions, Determination of the Order of Reaction and Reversible Reactions. UNIT-II (10 Hrs) Stereochemistry: Elements of Symmetry: Plane of Symmetry and Center of Symmetry, Alternating Axis of Symmetry, Simple Axis of Sy ...
... 2. Kinetic Studies: First Order Reactions, Second Order Reactions, Third Order Reactions, Determination of the Order of Reaction and Reversible Reactions. UNIT-II (10 Hrs) Stereochemistry: Elements of Symmetry: Plane of Symmetry and Center of Symmetry, Alternating Axis of Symmetry, Simple Axis of Sy ...
Chemistry 1 Lectures
... – Smaller charge is sometimes named as an ‘ic’ ion higher charge as an ‘ous’ ion – So in ferric chloride (FeCl2) iron ion is Fe2+ – Modern method is to indicate charge on the metal with Roman numerals – So FeCl2 is now named iron(II) chloride ...
... – Smaller charge is sometimes named as an ‘ic’ ion higher charge as an ‘ous’ ion – So in ferric chloride (FeCl2) iron ion is Fe2+ – Modern method is to indicate charge on the metal with Roman numerals – So FeCl2 is now named iron(II) chloride ...
Slide 1 - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
The Atom Power point - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
The Bio-Organometallic Chemistry of Technetium and Rhenium
... Given its central position in the periodic table, it is not surprising that the chemistry of technetium, the most widely used element in diagnostic medicine, is so diverse. Compounds of technetium exist in oxidation states from -I to +VII consisting of ligands that are as simple as hydride (H-) to m ...
... Given its central position in the periodic table, it is not surprising that the chemistry of technetium, the most widely used element in diagnostic medicine, is so diverse. Compounds of technetium exist in oxidation states from -I to +VII consisting of ligands that are as simple as hydride (H-) to m ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
Honors Chemistry
... concerned with the chemical properties of substances. Topics include: properties of matter; atomic theory, electron clouds and probability, periodic trends, chemical formulas, chemical reactions, chemical bonding kinetic theory, the gas laws, properties of solutions, reaction rates, acids and bases, ...
... concerned with the chemical properties of substances. Topics include: properties of matter; atomic theory, electron clouds and probability, periodic trends, chemical formulas, chemical reactions, chemical bonding kinetic theory, the gas laws, properties of solutions, reaction rates, acids and bases, ...
CHEM 1A General Chemistry I (1)
... 2. Use the periodic table to determine the structure of atoms of any element and of its isotopes. 3. Use the periodic table to determine the electronic configuration of any atom or ion. 4. Describe and discuss ionic bonding. 5. Describe and discuss covalent bonding. 6. Draw Lewis structures and appl ...
... 2. Use the periodic table to determine the structure of atoms of any element and of its isotopes. 3. Use the periodic table to determine the electronic configuration of any atom or ion. 4. Describe and discuss ionic bonding. 5. Describe and discuss covalent bonding. 6. Draw Lewis structures and appl ...
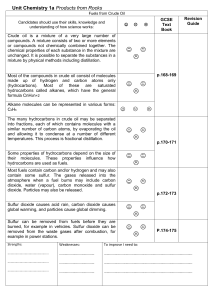
Unit_Chemistry_1a_Oil
... The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. Some properties of hydrocarbo ...
... The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. Some properties of hydrocarbo ...
Molecules, Compounds, and Chemical Equations (Chapter 3)
... • hard, brittle, high-melting crystalline solids • non-conductors in solid state, but conductors when molten • electrolytes -- separate into ions in aqueous solution Molecular compounds: • only weak attractive forces between uncharged molecules • generally low mp and bp • non-conductors of electrici ...
... • hard, brittle, high-melting crystalline solids • non-conductors in solid state, but conductors when molten • electrolytes -- separate into ions in aqueous solution Molecular compounds: • only weak attractive forces between uncharged molecules • generally low mp and bp • non-conductors of electrici ...
Chapt3
... hard, brittle, high-melting crystalline solids non-conductors in solid state, but conductors when molten electrolytes -- separate into ions in aqueous solution Molecular compounds: only weak attractive forces between uncharged molecules generally low mp and bp non-conductors of electrici ...
... hard, brittle, high-melting crystalline solids non-conductors in solid state, but conductors when molten electrolytes -- separate into ions in aqueous solution Molecular compounds: only weak attractive forces between uncharged molecules generally low mp and bp non-conductors of electrici ...
unit 2 - chemistry
... A. Basic definitions 1. matter – anything that occupies space and has mass 2. element – basic unit of all matter (109 total) a. 92 natural elements b. 4 basic – H, C, O, N, -96% of human mass Ca, P -> 99% (3%) K, S, Cl, Mg, I, FE, (16 other)1% -> ...
... A. Basic definitions 1. matter – anything that occupies space and has mass 2. element – basic unit of all matter (109 total) a. 92 natural elements b. 4 basic – H, C, O, N, -96% of human mass Ca, P -> 99% (3%) K, S, Cl, Mg, I, FE, (16 other)1% -> ...
Cluster Fragmentation and Catalysis
... such, they are usually thought of as bridging the gap between the gas and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catal ...
... such, they are usually thought of as bridging the gap between the gas and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catal ...
Document
... The number of atoms of the element in the compound is represented by its subscript. NOTE: ...
... The number of atoms of the element in the compound is represented by its subscript. NOTE: ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.