Summer Work
... that element. The atomic number gives the “identity “ of an element as well as its location on the Periodic Table. 5. No two different elements will have the ______________________ atomic number. 6. The ______________________ of an element is the average mass of an element’s naturally occurring atom ...
... that element. The atomic number gives the “identity “ of an element as well as its location on the Periodic Table. 5. No two different elements will have the ______________________ atomic number. 6. The ______________________ of an element is the average mass of an element’s naturally occurring atom ...
IB 1 CHEMISTRY
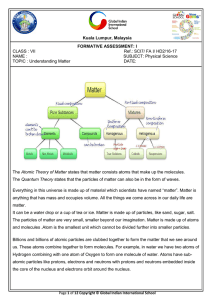
... Elements and compounds have a constant composition and are pure substances. Pure substances can combine to form a mixture. ...
... Elements and compounds have a constant composition and are pure substances. Pure substances can combine to form a mixture. ...
All chemical equations must be balanced, that is, they must have the
... coefficient of 2 in front of your hydrocarbon and proceed from there) There are times, however, that hydrocarbons do not combust completely; this is called incomplete combustion and the products are slightly different. You would always be told if the reaction was compete or incomplete, if nothing is ...
... coefficient of 2 in front of your hydrocarbon and proceed from there) There are times, however, that hydrocarbons do not combust completely; this is called incomplete combustion and the products are slightly different. You would always be told if the reaction was compete or incomplete, if nothing is ...
Cl Cl and
... to form a pair of electrons that goes around both atoms instead of around a single atom. How many electrons does each nucleus of chlorine “feel” going around it? How does this number compare to the number of electrons around the nucleus of the next noble gas? 18, the same. 12. Give, in your own word ...
... to form a pair of electrons that goes around both atoms instead of around a single atom. How many electrons does each nucleus of chlorine “feel” going around it? How does this number compare to the number of electrons around the nucleus of the next noble gas? 18, the same. 12. Give, in your own word ...
worksheer format 11-12
... Out of the three states of matter, solids are more common than liquid and gas. The main point in which a solid differs from the other two states is the fact that gases and liquid possess fluidity i.e. they can flow and are described as being fluid, while solids do not possess fluidity; instead they ...
... Out of the three states of matter, solids are more common than liquid and gas. The main point in which a solid differs from the other two states is the fact that gases and liquid possess fluidity i.e. they can flow and are described as being fluid, while solids do not possess fluidity; instead they ...
Class Activity
... crystalline form, boiling point, freezing point, and vapor pressure. Chemical Change: Chemical change is associated with change that results in a new substance with different properties. A chemical reaction is normally not easily reversed. For example, burning a piece of magnesium in oxygen produces ...
... crystalline form, boiling point, freezing point, and vapor pressure. Chemical Change: Chemical change is associated with change that results in a new substance with different properties. A chemical reaction is normally not easily reversed. For example, burning a piece of magnesium in oxygen produces ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
Ch. 02 - HCC Learning Web
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio • A compound has characteristics different from those of its elements ...
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio • A compound has characteristics different from those of its elements ...
Lab Stuff - WW-P 4
... Identify products, reactants, elements, symbols, coefficients, subscripts and compounds in the following equations: ...
... Identify products, reactants, elements, symbols, coefficients, subscripts and compounds in the following equations: ...
High School Physical Science Glossary
... ionic bond- type of chemical bond involving the transfer of electrons and the formation of ions; can often form between a metal and a non-metal ionic compound- compound where two or more ions are held next to each other by electrical attraction isotopes- atoms having the same number of protons (ato ...
... ionic bond- type of chemical bond involving the transfer of electrons and the formation of ions; can often form between a metal and a non-metal ionic compound- compound where two or more ions are held next to each other by electrical attraction isotopes- atoms having the same number of protons (ato ...
Topic 5 Reacting masses and chemical equations notes
... The -ide is added to the end to tell us that 2 elements have joined together, forming a compound. The metal always appears first in the name (if there is one). If the compound in made of two non-metals joined together, the element with the lower group number comes first. For naming compounds, hydrog ...
... The -ide is added to the end to tell us that 2 elements have joined together, forming a compound. The metal always appears first in the name (if there is one). If the compound in made of two non-metals joined together, the element with the lower group number comes first. For naming compounds, hydrog ...
AP Chemistry Review Assignment Brown and LeMay: Chemistry the
... The last part of this section, including how to determine the formula of a hydrate, and how to use combustion analyses to determine empirical formulas will be addressed early in the semester, probably Thurs., Aug. 20. 43. Give the empirical formula of each of the following compounds if a sample cont ...
... The last part of this section, including how to determine the formula of a hydrate, and how to use combustion analyses to determine empirical formulas will be addressed early in the semester, probably Thurs., Aug. 20. 43. Give the empirical formula of each of the following compounds if a sample cont ...
Chapter 1 The Periodic Table - Beck-Shop
... The Periodic Table Multiple Choice Items (1) The Periodic Table – Historical Development Question 1 Around 1800 a number of scientists observed that a pure compound always contains the same proportion of elements by mass. This was one of the observations used by Dalton when he formulated his atomic ...
... The Periodic Table Multiple Choice Items (1) The Periodic Table – Historical Development Question 1 Around 1800 a number of scientists observed that a pure compound always contains the same proportion of elements by mass. This was one of the observations used by Dalton when he formulated his atomic ...
Unit 1. Materials: Formulating Matter A. How do chemists describe
... This model, like all models, has some limitations. It is really only showing one small segment of the aluminum foil; the atoms extend outward from what is shown. The main point, though, is that we still only use a single symbol, Al, without any subscripts, to represent an entire collection of alumin ...
... This model, like all models, has some limitations. It is really only showing one small segment of the aluminum foil; the atoms extend outward from what is shown. The main point, though, is that we still only use a single symbol, Al, without any subscripts, to represent an entire collection of alumin ...
AP Chemistry Summer Assignment
... you complete as many online quizzes as possible, take detailed notes, and practice the items indicated in the packet. Completed work must be submitted by the appointed dates. Late work will not be accepted and could result in your removal from the class before school starts. The goal of these assign ...
... you complete as many online quizzes as possible, take detailed notes, and practice the items indicated in the packet. Completed work must be submitted by the appointed dates. Late work will not be accepted and could result in your removal from the class before school starts. The goal of these assign ...
Document
... brief description of process............................................................................................. ...
... brief description of process............................................................................................. ...
Begin Chemical Equations Practice
... (g) gas • (aq) aqueous or dissolved in water • (ppt) or ( ) means “a precipitate forms” • ( ) means “a gas bubbles off” ...
... (g) gas • (aq) aqueous or dissolved in water • (ppt) or ( ) means “a precipitate forms” • ( ) means “a gas bubbles off” ...
H2, N2, O2, F2, Cl2, Br2, I2
... 3. • Only change the coefficient ( the number in front of the formula ) when balancing. This tells us how many of each molecule or atom we have in the balanced equation. If there is no number in front, a " 1 " is there but we usually leave out the 1's. • Do not change subscripts to balance. They are ...
... 3. • Only change the coefficient ( the number in front of the formula ) when balancing. This tells us how many of each molecule or atom we have in the balanced equation. If there is no number in front, a " 1 " is there but we usually leave out the 1's. • Do not change subscripts to balance. They are ...
Chapter 10 - Chemical Reactions
... equal volumes of gas at STP have equal numbers of molecules FIXED formula for water: (twice as much H2) H2O SHOWED H and O diatomic (H2&O2) since two volumes of water produced New interpretation of chemical equation: coefficients in chemical equation give the volumes of gas ...
... equal volumes of gas at STP have equal numbers of molecules FIXED formula for water: (twice as much H2) H2O SHOWED H and O diatomic (H2&O2) since two volumes of water produced New interpretation of chemical equation: coefficients in chemical equation give the volumes of gas ...
2 Types of Chemical Bonds
... 2 Types of Chemical Bonds 1. Ionic Bond – gain or lose valence electrons • This is a chemical bond formed by the attraction between positive (+) and negative (-) ions. What types of elements form Ionic Bonds? Metal elements: • Lose valence electrons to form (+) ions • Easier to lose than gain to ge ...
... 2 Types of Chemical Bonds 1. Ionic Bond – gain or lose valence electrons • This is a chemical bond formed by the attraction between positive (+) and negative (-) ions. What types of elements form Ionic Bonds? Metal elements: • Lose valence electrons to form (+) ions • Easier to lose than gain to ge ...
Document
... represent elements and their ratios in each molecule of a compound Structural formula-shows how the atoms are connected in a molecule using symbols to represent atoms and lines to represent chemical bonds Ball and stick model-shows 3-D representation of molecules using different colored balls fo ...
... represent elements and their ratios in each molecule of a compound Structural formula-shows how the atoms are connected in a molecule using symbols to represent atoms and lines to represent chemical bonds Ball and stick model-shows 3-D representation of molecules using different colored balls fo ...
Revision topic 1-3
... Positive ions are smaller than their parent atoms (because of loss of the outer shell). Negative ions are larger than their parent atoms (because of increased electron repulsion by addition of electrons). The ionic radii decrease as a period is crossed from the left to the right (because of increase ...
... Positive ions are smaller than their parent atoms (because of loss of the outer shell). Negative ions are larger than their parent atoms (because of increased electron repulsion by addition of electrons). The ionic radii decrease as a period is crossed from the left to the right (because of increase ...
Name: Date: Period: _____ Unit 2 Notes, Part 1 – The Basics of
... organisms and non-living things are made up of matter. 2. Atoms are the smallest unit of matter. Each different type of atom represents an element (ex: hydrogen, oxygen, carbon). Scientists have created a chart called the periodic table of elements to organize elements by their atomic properties. 3. ...
... organisms and non-living things are made up of matter. 2. Atoms are the smallest unit of matter. Each different type of atom represents an element (ex: hydrogen, oxygen, carbon). Scientists have created a chart called the periodic table of elements to organize elements by their atomic properties. 3. ...
Matter, Mass and Weight
... Elements are substances that cannot be decomposed into simpler substances by physical or chemical means. Like all pure substances, it has its own set of physical and chemical properties. At room temperature, 75 elements are solids, 11 are gases, 2 (mercury and bromine) are liquids. Each element is r ...
... Elements are substances that cannot be decomposed into simpler substances by physical or chemical means. Like all pure substances, it has its own set of physical and chemical properties. At room temperature, 75 elements are solids, 11 are gases, 2 (mercury and bromine) are liquids. Each element is r ...