EKSIKA JOINT EVALUATION TEST. Kenya Certificate
... Write chemical equation for the formation of the following compound.(3mks) a. ...
... Write chemical equation for the formation of the following compound.(3mks) a. ...
Chapter 4 The Structure of Matter
... attraction between positively charged ions and the electrons around them. • 2. Metal to metal • a. example: Cu ...
... attraction between positively charged ions and the electrons around them. • 2. Metal to metal • a. example: Cu ...
educator exam series
... (h) What is the name given to the reaction that takes place when sodium carbonate was addedto the filtrate? (1mkss) 7.(a) In an experiment to investigate the properties of hydrogen, a student set up as follows. ...
... (h) What is the name given to the reaction that takes place when sodium carbonate was addedto the filtrate? (1mkss) 7.(a) In an experiment to investigate the properties of hydrogen, a student set up as follows. ...
Midterm Review 4
... 2.00cm3. What is its density? a) 8.22g/cm3 b) 2.06g/cm3 c) 0.49g/cm3 d) not enough information 15. A student estimated a mass to be 250g, but upon carefully measuring it, found it to be 240g, What is the percent error of the estimated mass? a) 4.0% b) 4.2% c) -4.0% d) -4.2% 16. The number of electro ...
... 2.00cm3. What is its density? a) 8.22g/cm3 b) 2.06g/cm3 c) 0.49g/cm3 d) not enough information 15. A student estimated a mass to be 250g, but upon carefully measuring it, found it to be 240g, What is the percent error of the estimated mass? a) 4.0% b) 4.2% c) -4.0% d) -4.2% 16. The number of electro ...
AP Chemistry Ch. 3 Sections 3.7-3.8 Notes Chemical Equations
... All atoms present in the reactants must be accounted for among the products. In other words, there must be the same number of each type of atom on the product side and on the reactant side of the arrow. Known as balancing a chemical equation. CH4 + O2 → CO2 + H2O This equation is not balanced. Notic ...
... All atoms present in the reactants must be accounted for among the products. In other words, there must be the same number of each type of atom on the product side and on the reactant side of the arrow. Known as balancing a chemical equation. CH4 + O2 → CO2 + H2O This equation is not balanced. Notic ...
Matter
... In a system of two or more phases, each phase is a visibly different part which has different properties The variation in properties may be : - different physical properties in each phase - different chemical properties - different physical and chemical properties ...
... In a system of two or more phases, each phase is a visibly different part which has different properties The variation in properties may be : - different physical properties in each phase - different chemical properties - different physical and chemical properties ...
Chemistry Midterm Review 2006
... 3. Differentiate characteristics of pure substances and mixtures. 4. Pure substances can be broken down into________________ and ___________________________. 5. Differentiate characteristics of an element and a compound. State examples. 6. Mixtures can be classified as _______________________ and __ ...
... 3. Differentiate characteristics of pure substances and mixtures. 4. Pure substances can be broken down into________________ and ___________________________. 5. Differentiate characteristics of an element and a compound. State examples. 6. Mixtures can be classified as _______________________ and __ ...
File
... 20. Element whose atoms lose electrons in chemical reactions to become positive ions. 21. Groups 3-12 on the periodic table. 22. Scientist who performed the gold foil experiment, and concluded that an atom must be composed of mostly empty space with a small, dense, positively-charged nucleus. 23. An ...
... 20. Element whose atoms lose electrons in chemical reactions to become positive ions. 21. Groups 3-12 on the periodic table. 22. Scientist who performed the gold foil experiment, and concluded that an atom must be composed of mostly empty space with a small, dense, positively-charged nucleus. 23. An ...
Chemistry - Edgbarrow School
... metals and acids, as examples of Table carbon dioxide and breaking of bonds chemical reactions ...
... metals and acids, as examples of Table carbon dioxide and breaking of bonds chemical reactions ...
New substances are formed by chemical reactions. When elements
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
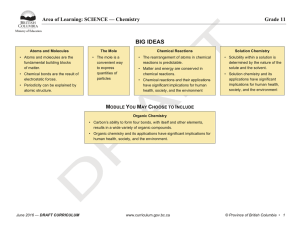
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Apply First Peoples perspectives and knowledge, other ways of knowing, and local knowledge as sources of information • Seek and analyze patterns, trends, and connections in data, including describing relationships between variables, performing calculations, and identifying ...
... • Apply First Peoples perspectives and knowledge, other ways of knowing, and local knowledge as sources of information • Seek and analyze patterns, trends, and connections in data, including describing relationships between variables, performing calculations, and identifying ...
Answer key
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
Chemical Reactions
... To make a quantity that is measurable in lab, we use the mole. In chemistry one mole is equal to 6.022 x 1023 particles (Avogadro’s number). The gram formula mass of any compound is the mass of 1 mole of the compound in grams. 1 mole = 6.0022 x 1023 is similar to 12 eggs = 1 dozen 52 weeks = 1 y ...
... To make a quantity that is measurable in lab, we use the mole. In chemistry one mole is equal to 6.022 x 1023 particles (Avogadro’s number). The gram formula mass of any compound is the mass of 1 mole of the compound in grams. 1 mole = 6.0022 x 1023 is similar to 12 eggs = 1 dozen 52 weeks = 1 y ...
File
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
Chem MCQ for Class-9th
... c. Do not change from left to right in a period d. Decrease from top to bottom in a group 2. The amount of energy given out when an electron is added to an atom is called: a. Lattice entery b. ionization entergy c. electronegativity d. electron affinity 3. Mendeleev Periodic Table was based upon the ...
... c. Do not change from left to right in a period d. Decrease from top to bottom in a group 2. The amount of energy given out when an electron is added to an atom is called: a. Lattice entery b. ionization entergy c. electronegativity d. electron affinity 3. Mendeleev Periodic Table was based upon the ...
CHEM 11 Practice Exam 2
... B) p electrons C) bonding electrons D) valence electrons E) none of the above 2) Which element has the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s1? A) Al B) Ca C) K D) Na E) none of the above 3) What is the maximum number of electrons in the 3rd energy level? A) 2 B) 8 C) 18 D) 32 E) n ...
... B) p electrons C) bonding electrons D) valence electrons E) none of the above 2) Which element has the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s1? A) Al B) Ca C) K D) Na E) none of the above 3) What is the maximum number of electrons in the 3rd energy level? A) 2 B) 8 C) 18 D) 32 E) n ...
Masterton and Hurley Chapter 3
... moles of all of the answers to #3 5. *If the answers to #4 are whole numbers, these are the subscripts in the empirical formula. * If any of the answers to #4 is not a whole number, convert all answers to a common fraction. Multiply each fraction by the denominator resulting in a whole number and th ...
... moles of all of the answers to #3 5. *If the answers to #4 are whole numbers, these are the subscripts in the empirical formula. * If any of the answers to #4 is not a whole number, convert all answers to a common fraction. Multiply each fraction by the denominator resulting in a whole number and th ...
Table showing examples of Complex ions with their bond
... that causes the decrease in radius. The decrease in radius coupled with as increasing relative atomic mass causes an increase in density and a decrease in atomic volume in passing from Sc to Ni. There is increase in electronegativity in passing from Sc to Ni. Oxidation State (O.S.) With the exceptio ...
... that causes the decrease in radius. The decrease in radius coupled with as increasing relative atomic mass causes an increase in density and a decrease in atomic volume in passing from Sc to Ni. There is increase in electronegativity in passing from Sc to Ni. Oxidation State (O.S.) With the exceptio ...
- Catalyst
... 4. The ______molar mass______________________ of an element has the units g/mole. 5. Smallest unit of an element is a(n) ____atom_____________________________. 6. The ___chemical symbol_________ of an element is one or two letters found on the periodic table. 7. Isotopes have the same number ...
... 4. The ______molar mass______________________ of an element has the units g/mole. 5. Smallest unit of an element is a(n) ____atom_____________________________. 6. The ___chemical symbol_________ of an element is one or two letters found on the periodic table. 7. Isotopes have the same number ...
CHEM_S1CourseReview_2011
... What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment ...
... What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment ...
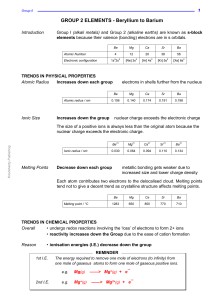
GROUP 2 ELEMENTS - Beryllium to Barium
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
Unit 1: Building Blocks Homework
... In the Unit 1 PPA, “Effect of concentration on reaction rate”, the reaction between sodium persulphate and potassium iodide solutions is studied. The formula for the persulphate ion is S2O82- . Write the formula for sodium persulphate. ...
... In the Unit 1 PPA, “Effect of concentration on reaction rate”, the reaction between sodium persulphate and potassium iodide solutions is studied. The formula for the persulphate ion is S2O82- . Write the formula for sodium persulphate. ...
Unit 2 Review Game
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...