Chemical Reactions
... 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: • The numbers of atoms on both sides of the equation are now balanced. • The coefficie ...
... 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: • The numbers of atoms on both sides of the equation are now balanced. • The coefficie ...
CHEMISTRY IM 06 SYLLABUS 1
... volume for gases will also be given when required. Syllabus Chemistry is an experimental science and it is essential that students spend time in a laboratory to see for themselves how chemists work. It may not be possible for students following this course to participate actively in laboratory work: ...
... volume for gases will also be given when required. Syllabus Chemistry is an experimental science and it is essential that students spend time in a laboratory to see for themselves how chemists work. It may not be possible for students following this course to participate actively in laboratory work: ...
Practice Multiple Choice Questions for the Chemistry Final Exam
... 65. The reaction Mg(s) + 2HCI(aq) H2(g) + MgCl2(aq) is a a) composition reaction b) decomposition reaction. c) single-replacement reaction. d) double-replacement reaction. 66. The reaction Pb(NO3)2(aq) + 2KI(aq) PbI2(S) + 2KNO3(aq) is a a) double-replacement reaction. b) synthesis reaction. c) d ...
... 65. The reaction Mg(s) + 2HCI(aq) H2(g) + MgCl2(aq) is a a) composition reaction b) decomposition reaction. c) single-replacement reaction. d) double-replacement reaction. 66. The reaction Pb(NO3)2(aq) + 2KI(aq) PbI2(S) + 2KNO3(aq) is a a) double-replacement reaction. b) synthesis reaction. c) d ...
Cosmetology Learning Module 12
... matter and how matter changes under different chemical conditions Organic Chemistry – is the study of substances that contain carbon All living things are made up of compounds that contain carbon Organic compounds will burn ...
... matter and how matter changes under different chemical conditions Organic Chemistry – is the study of substances that contain carbon All living things are made up of compounds that contain carbon Organic compounds will burn ...
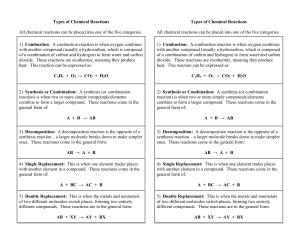
Types of Chemical Reactions
... general form of: A + BC → AC + B 5) Double Replacement: This is when the metals and nonmetals of two different molecules switch places, forming two entirely different compounds. These reactions are in the general form: ...
... general form of: A + BC → AC + B 5) Double Replacement: This is when the metals and nonmetals of two different molecules switch places, forming two entirely different compounds. These reactions are in the general form: ...
Chemistry - StudyTime NZ
... Neither Oxygen nor Magnesium have full valence electron shells. Because of this, they must each lose or gain electrons in order to become stable. Oxygen has 8 electrons and hence an electron arrangement ...
... Neither Oxygen nor Magnesium have full valence electron shells. Because of this, they must each lose or gain electrons in order to become stable. Oxygen has 8 electrons and hence an electron arrangement ...
Year 9 Science revison _15-16_ end of year CHEM
... iv) The compound RbSO4 dissolves in water, but the element Rb doesn’t. Why ? The compound is made up of ions bonded together. When it dissolves in water, these ions separate from each other. They are charged (+ and -) and able to attract to and bond with water. This is why the ionic compound CAN dis ...
... iv) The compound RbSO4 dissolves in water, but the element Rb doesn’t. Why ? The compound is made up of ions bonded together. When it dissolves in water, these ions separate from each other. They are charged (+ and -) and able to attract to and bond with water. This is why the ionic compound CAN dis ...
1 - New Age International
... (d) It refers to the amount of a substance that contains as many elementary particles (atoms, molecules or ions) as there are atoms in 12 grams of carbon-12. 8. The gram molecular weight of a substance is (a) the weight of a molecule of the substance in grams. (b) the sum of the weights of all the a ...
... (d) It refers to the amount of a substance that contains as many elementary particles (atoms, molecules or ions) as there are atoms in 12 grams of carbon-12. 8. The gram molecular weight of a substance is (a) the weight of a molecule of the substance in grams. (b) the sum of the weights of all the a ...
Stars and Elements
... HOW CAN LOOKING AT THE SAME INFORMATION FROM DIFFERENT PERSPECTIVES PAVE THE WAY FOR PROGRESS? ...
... HOW CAN LOOKING AT THE SAME INFORMATION FROM DIFFERENT PERSPECTIVES PAVE THE WAY FOR PROGRESS? ...
Unit 2: Chemical Reactions
... • A chemical formula is an abbreviation for a chemical compound using chemical symbols and numbers. • The subscript number tells how many atoms of the element are present in the compound • Example: CO2 = Carbon Dioxide – Di = 2 – 1 Carbon atom and 2 oxygen atoms ...
... • A chemical formula is an abbreviation for a chemical compound using chemical symbols and numbers. • The subscript number tells how many atoms of the element are present in the compound • Example: CO2 = Carbon Dioxide – Di = 2 – 1 Carbon atom and 2 oxygen atoms ...
Atomic Theory Review
... Atoms of a given element are identical in size, mass and other properties. Atoms cannot be divided, created or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. Atoms may be spl ...
... Atoms of a given element are identical in size, mass and other properties. Atoms cannot be divided, created or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. Atoms may be spl ...
Final Exam Practice
... a. Friction is a force that resists motion. b. Friction should always be removed. c. Friction only occurs between solids. d. Oils and other lubricants totally eliminate friction. ____ 22. ...
... a. Friction is a force that resists motion. b. Friction should always be removed. c. Friction only occurs between solids. d. Oils and other lubricants totally eliminate friction. ____ 22. ...
Name: (1 of 2) Math Set # 13 Protons,
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
AP Chap 2
... • Matter is made up of elements • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio • A compound has characteristics different from those of its elements ...
... • Matter is made up of elements • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio • A compound has characteristics different from those of its elements ...
Chapter 3
... John Dalton proposed that all matter is composed of very small things which he called atoms. This was not completely new concept as the ancient Greeks had proposed that all matter is composed of small, indivisible objects. When Dalton proposed his model electrons and the nucleus were unknown. ...
... John Dalton proposed that all matter is composed of very small things which he called atoms. This was not completely new concept as the ancient Greeks had proposed that all matter is composed of small, indivisible objects. When Dalton proposed his model electrons and the nucleus were unknown. ...
Chapter-1-Intro - Mister Chemistry Welcomes You!
... a substance is form of matter that has a definite or constant composition and distinct properties, for example water ammonia table sugar gold oxygen ...
... a substance is form of matter that has a definite or constant composition and distinct properties, for example water ammonia table sugar gold oxygen ...
Document
... Matter is the physical material of the universe. Matter is anything that occupies space (volume) and ...
... Matter is the physical material of the universe. Matter is anything that occupies space (volume) and ...
Unit B Chemistry Unit study guide
... halogens, noble gases as well as metals vs nonmetals Why are lanthanides and actinides on bottom? What are the only two liquids? Where are the gasses? Which element is in a group of its own? Which element is needed for substances to burn? Mendeleev did such a great job creating his periodic table be ...
... halogens, noble gases as well as metals vs nonmetals Why are lanthanides and actinides on bottom? What are the only two liquids? Where are the gasses? Which element is in a group of its own? Which element is needed for substances to burn? Mendeleev did such a great job creating his periodic table be ...
Elements, Compounds and Mixtures.
... The properties of a mixture are The properties of a compound similar to those of the substances are different to those of the in a mixture. elements which reacted to form it. There are practically no energy Heat is usually given out or changes when a mixture is made taken in when a compound is forme ...
... The properties of a mixture are The properties of a compound similar to those of the substances are different to those of the in a mixture. elements which reacted to form it. There are practically no energy Heat is usually given out or changes when a mixture is made taken in when a compound is forme ...
SAT Practice Test 3
... NH3 is a polar substance Water boils when the vapor pressure of the water is equal to the atmospheric pressure In an exothermic reaction the products have less potential energy than the reactants Pressure and volume have a direct relationship Ethane, has as many hydrogen atoms bonded to the carbon a ...
... NH3 is a polar substance Water boils when the vapor pressure of the water is equal to the atmospheric pressure In an exothermic reaction the products have less potential energy than the reactants Pressure and volume have a direct relationship Ethane, has as many hydrogen atoms bonded to the carbon a ...
Unit 3 - Princeton High School
... _______________ _____ __________, that matter could not be created or destroyed. Then ___________ proposed, in his law of ____________ _____________, that the ratio of the masses of elements in any given compound is always the same. The law of _____________ ______________ , proposed soon after, stat ...
... _______________ _____ __________, that matter could not be created or destroyed. Then ___________ proposed, in his law of ____________ _____________, that the ratio of the masses of elements in any given compound is always the same. The law of _____________ ______________ , proposed soon after, stat ...
Chemistry Final Exam Study Guide
... d. observation ____ 26. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 27. Which of the following would a chemist be most likely to study? a. a leaf floating on water c. a leaf being blown by the wind b. a leaf changing colo ...
... d. observation ____ 26. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 27. Which of the following would a chemist be most likely to study? a. a leaf floating on water c. a leaf being blown by the wind b. a leaf changing colo ...
BAT
... g. When this reaction was carried out in the lab, 3.487 grams of product was obtained. What is the percent yield of this reaction? Chapter 10 Important topics: kinetic molecular theory, gas laws – Boyle, Charles, Gay-Lussac, combined, Dalton ...
... g. When this reaction was carried out in the lab, 3.487 grams of product was obtained. What is the percent yield of this reaction? Chapter 10 Important topics: kinetic molecular theory, gas laws – Boyle, Charles, Gay-Lussac, combined, Dalton ...
Excited Elements - Light Emission Spectroscopy
... gas-filled glass tube. Gases under low pressure and excited by an electrical discharge give off light in characteristic wavelengths. The emitted light is passed through a spectroscope, which breaks light into its components for analysis. A gas viewed through a spectroscope, such as the one shown in ...
... gas-filled glass tube. Gases under low pressure and excited by an electrical discharge give off light in characteristic wavelengths. The emitted light is passed through a spectroscope, which breaks light into its components for analysis. A gas viewed through a spectroscope, such as the one shown in ...