Mixtures, Pure Substance and Isotopes
... • Chemists like lists, rules and categories • As a result chemists like to classify matter • Matter is classified based on its composition ...
... • Chemists like lists, rules and categories • As a result chemists like to classify matter • Matter is classified based on its composition ...
Day 72 TYPES OF CHEMICAL REACTIONS
... a) write a description of the reaction type on the left side b) an example of that type of reaction using elements/compounds and an example of the reaction type using the letters A, B, C and/or D on the right c) And three examples of the reaction ...
... a) write a description of the reaction type on the left side b) an example of that type of reaction using elements/compounds and an example of the reaction type using the letters A, B, C and/or D on the right c) And three examples of the reaction ...
Chemical Equations and Tests for anions
... The arrow sign means “react to give” the + sign means “and” ...
... The arrow sign means “react to give” the + sign means “and” ...
Unit B review - mvhs
... Multiple Choice: Most of the following are actual questions from previous AP Exams. You may work on them alone or with partners, but try to complete them using only a periodic table and calculator, if necessary. These 30 questions should take you about 30 minutes to finish. ...
... Multiple Choice: Most of the following are actual questions from previous AP Exams. You may work on them alone or with partners, but try to complete them using only a periodic table and calculator, if necessary. These 30 questions should take you about 30 minutes to finish. ...
110 exam i material
... Metric metric conversion factors are exact numbers and have an infinite number of significant figures English english conversion factors are exact numbers and have an infinite number of significant figures English metric conversion factors are measured numbers and have a finite number of signi ...
... Metric metric conversion factors are exact numbers and have an infinite number of significant figures English english conversion factors are exact numbers and have an infinite number of significant figures English metric conversion factors are measured numbers and have a finite number of signi ...
ppt Sc10 Review Notes
... same as before …look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “xH2O” where x is the number given in the prefix eg) iron (III) nitrate nonahydrate = Fe(NO3)39H2O sodium chlorate tetrahydrate = NaClO34H2O nickel (II) sulphite heptahydrate = ...
... same as before …look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “xH2O” where x is the number given in the prefix eg) iron (III) nitrate nonahydrate = Fe(NO3)39H2O sodium chlorate tetrahydrate = NaClO34H2O nickel (II) sulphite heptahydrate = ...
The Atom - dsapresents.org
... 1. All elements are composed of tiny indivisible particles called atoms 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms can physically mix together or can chemically combine in simple whole number ratios. 4. Chemical re ...
... 1. All elements are composed of tiny indivisible particles called atoms 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms can physically mix together or can chemically combine in simple whole number ratios. 4. Chemical re ...
9/6/12 - Note: Once it is downloaded, click SET
... - A chemical property of many substances is their reactivity with oxygen. o Rusting, corrosion - Some substances break down into new substances when heated Classifying Matter - An atom is the smallest unit of an element that maintains the properties of that element. - Matter exists in many different ...
... - A chemical property of many substances is their reactivity with oxygen. o Rusting, corrosion - Some substances break down into new substances when heated Classifying Matter - An atom is the smallest unit of an element that maintains the properties of that element. - Matter exists in many different ...
First Semester complete review with answers
... Element- 1 kind of atom (all the atoms are alike), pure substance, organized on Periodic Table OF ELEMENTS, identified by the atomic ‘protomic’ number ...
... Element- 1 kind of atom (all the atoms are alike), pure substance, organized on Periodic Table OF ELEMENTS, identified by the atomic ‘protomic’ number ...
Chapter 2 ATOMS AND ELEMENTS
... called atoms According to Democritus, atoms could not be broken into smaller particles. ...
... called atoms According to Democritus, atoms could not be broken into smaller particles. ...
Chapter 3 - Stoichiometry
... Treat % as grams and convert grams to moles using the mass from the PTE Find the smallest whole number ratio of atoms (multiply by an integer to make them whole numbers). A compound contains 63.5% Silver, 8.2% Nitrogen and 28.2% Oxygen. What is the empirical formula for this compound? Ag = 63.5 ...
... Treat % as grams and convert grams to moles using the mass from the PTE Find the smallest whole number ratio of atoms (multiply by an integer to make them whole numbers). A compound contains 63.5% Silver, 8.2% Nitrogen and 28.2% Oxygen. What is the empirical formula for this compound? Ag = 63.5 ...
I. scientific notation. – a shorthand that scientists use when dealing
... 2. in chemical changes (reaction stoichiometry) Dalton’s Atomic Theory – summarized experimental observations and interpretations in the nature of atoms: 1. an element is composed of extremely small, indivisible particles called atoms 2. Atoms cannot be created, destroyed, or transformed into atoms ...
... 2. in chemical changes (reaction stoichiometry) Dalton’s Atomic Theory – summarized experimental observations and interpretations in the nature of atoms: 1. an element is composed of extremely small, indivisible particles called atoms 2. Atoms cannot be created, destroyed, or transformed into atoms ...
Unit 2: Mixture and Matter Study Guide Ch 2 Vocab to know: Matter
... Chemical property Physical change Chemical change Intensive Homogenous Filtration ...
... Chemical property Physical change Chemical change Intensive Homogenous Filtration ...
Chemical Reactions
... Chemical Reactions • New Substances produced by changing the way atoms are arranged • Physical Changes ≠ Chemical Reaction • Evidence ...
... Chemical Reactions • New Substances produced by changing the way atoms are arranged • Physical Changes ≠ Chemical Reaction • Evidence ...
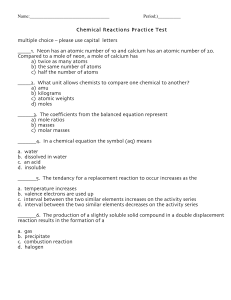
Chemical Reactions Practice Test
... _____1. Neon has an atomic number of 10 and calcium has an atomic number of 20. Compared to a mole of neon, a mole of calcium has a) twice as many atoms b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c ...
... _____1. Neon has an atomic number of 10 and calcium has an atomic number of 20. Compared to a mole of neon, a mole of calcium has a) twice as many atoms b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c ...
Ch 2 notes
... 2. All atoms of a given element are identical (specifically in their masses). 3. Atoms of any given element are different than atoms of any other element (specifically in their masses). 4. A given compound always has the same relative numbers (whole number ratios) and kinds of atoms. Note: not all t ...
... 2. All atoms of a given element are identical (specifically in their masses). 3. Atoms of any given element are different than atoms of any other element (specifically in their masses). 4. A given compound always has the same relative numbers (whole number ratios) and kinds of atoms. Note: not all t ...
Chemistry - Solutions
... • Solubility: the amount of a substance that will dissolve in a given amount of solvent to form a saturated solution at a given temperature • Solubility depends on RANDOM MOLECULAR MOTION, which is affected by temperature, pressure and surface ...
... • Solubility: the amount of a substance that will dissolve in a given amount of solvent to form a saturated solution at a given temperature • Solubility depends on RANDOM MOLECULAR MOTION, which is affected by temperature, pressure and surface ...
June review January 2012 part A
... to the kinetic molecular theory? (Hint: items 55,56 of 200 ways..) (1) The gas particles are arranged in a regular geometric pattern. (2) The gas particles are in random, constant, straight-line motion (3) The gas particles are separated by very small distances, relative to ...
... to the kinetic molecular theory? (Hint: items 55,56 of 200 ways..) (1) The gas particles are arranged in a regular geometric pattern. (2) The gas particles are in random, constant, straight-line motion (3) The gas particles are separated by very small distances, relative to ...
Chem A Week 2 Matter Notes
... from place to place, and slide past each other in constant motion. However, they essentially contact remain in __________ with each other at all times as the slide around, so there is not much ...
... from place to place, and slide past each other in constant motion. However, they essentially contact remain in __________ with each other at all times as the slide around, so there is not much ...
The Language of Chemistry
... • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary m ...
... • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary m ...
Chemistry I Exam
... The “bright lines” making up the spectra of excited gaseous atoms help to identify the various energy levels of these atoms. Which statement does NOT help to explain the observed line spectra? A. Electrons tend to drop to the lowest available energy levels in an atom. B. Each frequency of light corr ...
... The “bright lines” making up the spectra of excited gaseous atoms help to identify the various energy levels of these atoms. Which statement does NOT help to explain the observed line spectra? A. Electrons tend to drop to the lowest available energy levels in an atom. B. Each frequency of light corr ...
chapter 6 sec 2 resonance structure
... H2O is a molecule which makes H2O a molecular compound and a molecular formula. But H2O is also a chemical formula because we use atomic symbols and subscripts to describe it. ...
... H2O is a molecule which makes H2O a molecular compound and a molecular formula. But H2O is also a chemical formula because we use atomic symbols and subscripts to describe it. ...
Chemistry Midterm Review Sheet
... 1) Balance the following chemical equations and name each product and reactant: a) Ca(OH)2 + HCl CaCl2 + H2O b) Fe2O3 + C Fe + CO2 c) P4O10 + H2O H3PO4 d) Al + H2SO4 Al2(SO4)3 + H2 Write balanced net ionic equations for the reactions that may occur when each of the following pairs is mixed. ...
... 1) Balance the following chemical equations and name each product and reactant: a) Ca(OH)2 + HCl CaCl2 + H2O b) Fe2O3 + C Fe + CO2 c) P4O10 + H2O H3PO4 d) Al + H2SO4 Al2(SO4)3 + H2 Write balanced net ionic equations for the reactions that may occur when each of the following pairs is mixed. ...