å¾è湿çå¦
... • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make the ...
... • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make the ...
2015 Dr. Jay L. Wile, All rights reserved.
... 9. If a chemist reacts 6.4 g of copper with 1.6 grams of oxygen, cupric oxide is made. It is composed of one copper atom and one oxygen atom. However, copper and oxygen can also combine to make cuprous oxide, which is made of two copper atoms and one oxygen atom. Suppose you react 1.6 grams of oxyge ...
... 9. If a chemist reacts 6.4 g of copper with 1.6 grams of oxygen, cupric oxide is made. It is composed of one copper atom and one oxygen atom. However, copper and oxygen can also combine to make cuprous oxide, which is made of two copper atoms and one oxygen atom. Suppose you react 1.6 grams of oxyge ...
Chemistry 133 Problem Set Introduction
... 1.27 Using the table inside the front cover of this textbook, list the symbol for each of the following elements: (a) hydrogen (b) lithium (c) aluminum (d) xenon (e) samarium (f) iron (g) copper (h) sodium (i) mercury (j) lead 1.28 Use the table inside the cover of this textbook, first, to classify ...
... 1.27 Using the table inside the front cover of this textbook, list the symbol for each of the following elements: (a) hydrogen (b) lithium (c) aluminum (d) xenon (e) samarium (f) iron (g) copper (h) sodium (i) mercury (j) lead 1.28 Use the table inside the cover of this textbook, first, to classify ...
Table of Contents - slccscience`s Home Page
... elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a separate discipline, but there is a very good reason for this. There are about 1.5 million known inorganic co ...
... elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a separate discipline, but there is a very good reason for this. There are about 1.5 million known inorganic co ...
Chemistry Essentials For Dummies
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
Integrated Physics and Chemistry
... atoms transfer their valence electrons to form ionic bonds, while other atoms share valence electrons to form covalent bonds; Differentiate between ionic, covalent, and metallic bonds; Compare the properties of substances with different types of bonds Name simple ionic and covalent compounds; Predic ...
... atoms transfer their valence electrons to form ionic bonds, while other atoms share valence electrons to form covalent bonds; Differentiate between ionic, covalent, and metallic bonds; Compare the properties of substances with different types of bonds Name simple ionic and covalent compounds; Predic ...
AP Chemistry - Notes
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... Chemistry is the study of matter and the changes it undergoes. ...
... Chemistry is the study of matter and the changes it undergoes. ...
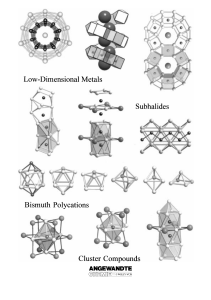
From the Metal to the Molecule
... knowledge regarding the gas-phase species. The possibility of observing new ternary bismuth subhalides in the ternary systems, where new compounds have already been discovered, as well as those, which up to now have looked less promising, cannot be ruled out. The chemical properties of the binary an ...
... knowledge regarding the gas-phase species. The possibility of observing new ternary bismuth subhalides in the ternary systems, where new compounds have already been discovered, as well as those, which up to now have looked less promising, cannot be ruled out. The chemical properties of the binary an ...
4) What is the term for the procedure of collecting data and recording
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...
Electronic Student Book Glossary and Index
... camera lightproof box with a lens at one end to form a real, inverted image on a light detector or on a light-sensitive plate or film (484) cancer cell cell that divides uncontrollably; develops when a mutation occurs in the cell that affects how that cell divides ...
... camera lightproof box with a lens at one end to form a real, inverted image on a light detector or on a light-sensitive plate or film (484) cancer cell cell that divides uncontrollably; develops when a mutation occurs in the cell that affects how that cell divides ...
To do List
... He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. ...
... He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. ...
Empirical Formula, Molecular Formula, Percent Composition
... 1. The molecular formula indicates the types and number of atoms that make up a chemical compound. The chemical (molecular) formula is a multiple of a much simpler formula called the empirical formula. The empirical formula is simply the lowest reduced subscripts that make up a molecular formula. Fo ...
... 1. The molecular formula indicates the types and number of atoms that make up a chemical compound. The chemical (molecular) formula is a multiple of a much simpler formula called the empirical formula. The empirical formula is simply the lowest reduced subscripts that make up a molecular formula. Fo ...
ANSWERS Problem Set 5a – Chemical Reactions
... 34) Define “Reactant in Excess” and “Limiting Reactant.” Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemi ...
... 34) Define “Reactant in Excess” and “Limiting Reactant.” Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemi ...
NOBLE-GAS CHEMISTRY
... 2.3. Very cold and exotic compounds with noble gas–metal bonds, and an unexpected warming It is now known that noble-gas atoms do not bind exclusively to nonmetals, but they may also form bonds to metals (see Fig. 4). This was realized, however, only in 1983 despite the many preceding inorganic and ...
... 2.3. Very cold and exotic compounds with noble gas–metal bonds, and an unexpected warming It is now known that noble-gas atoms do not bind exclusively to nonmetals, but they may also form bonds to metals (see Fig. 4). This was realized, however, only in 1983 despite the many preceding inorganic and ...
When wood, paper, and wax are burned, they ap
... In this chapter we will use what we have learned about chemical structure and formulas in studying the mass relationships of atoms and molecules. These relationships in turn will help us to explain the composition of compounds and the ways in which the composition changes. The mass of an atom is rel ...
... In this chapter we will use what we have learned about chemical structure and formulas in studying the mass relationships of atoms and molecules. These relationships in turn will help us to explain the composition of compounds and the ways in which the composition changes. The mass of an atom is rel ...
Naming Compounds - Kowenscience.com
... Naming ionic compounds containing polyatomic ions follows rules similar to those for binary compounds. ...
... Naming ionic compounds containing polyatomic ions follows rules similar to those for binary compounds. ...
Exam Edge Digital
... (c) Name the series of coloured lines in the line emission spectrum of hydrogen corresponding to transitions of electrons from higher energy levels to the second (n = 2) energy level. (e) Distinguish between an atomic orbital and a sub-level. ...
... (c) Name the series of coloured lines in the line emission spectrum of hydrogen corresponding to transitions of electrons from higher energy levels to the second (n = 2) energy level. (e) Distinguish between an atomic orbital and a sub-level. ...
Class-XII, Summer assignment
... with a borate buffer (pH 9.2), iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method for estimating O3 gas. ...
... with a borate buffer (pH 9.2), iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method for estimating O3 gas. ...
Stoichiometry: Calculations with Chemical Formulas and
... Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten ...
... Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten ...
Chapter 3 PowerPoint Presentation
... • As many new elements were being discovered in the early nineteenth century, chemists began to see patterns of similarities in the chemical and physical properties of particular sets of elements. • Several schemes for organizing the elements on the basis of these similarities were proposed, with va ...
... • As many new elements were being discovered in the early nineteenth century, chemists began to see patterns of similarities in the chemical and physical properties of particular sets of elements. • Several schemes for organizing the elements on the basis of these similarities were proposed, with va ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... CHEM.A.2.2.3 – Explain the relationship between the electron configurations and the atomic structure of a given atom or ion (e.g., energy levels and/or orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). ...
... CHEM.A.2.2.3 – Explain the relationship between the electron configurations and the atomic structure of a given atom or ion (e.g., energy levels and/or orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). ...
Chapter 3 Mass Relationships in Chemical Reactions
... 50. An average atom of uranium (U) is approximately how many times heavier than an atom of potassium? A. B. C. D. E. ...
... 50. An average atom of uranium (U) is approximately how many times heavier than an atom of potassium? A. B. C. D. E. ...