2010 Released SOL
... Precision is how close your numbers are to each other, so the 113°C is irrelevant to this problem. (It would only be used for accuracy; how close the group is to the correct answer). Plus, make sure you are reading down the columns for each group. But the group with the most consistent (or precis ...
... Precision is how close your numbers are to each other, so the 113°C is irrelevant to this problem. (It would only be used for accuracy; how close the group is to the correct answer). Plus, make sure you are reading down the columns for each group. But the group with the most consistent (or precis ...
Stoichiometry: Calculations with Chemical Formulas and
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Chemistry Senior External Syllabus 1998
... Science is a cultural endeavour. It incorporates a way of thinking as well as a body of knowledge. Characteristically, it adopts an empirical approach to the search for natural explanations of phenomena observed in the universe. Although the study of science in the senior school is divided into a nu ...
... Science is a cultural endeavour. It incorporates a way of thinking as well as a body of knowledge. Characteristically, it adopts an empirical approach to the search for natural explanations of phenomena observed in the universe. Although the study of science in the senior school is divided into a nu ...
H2 Chemistry Syllabus (9729)
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The submicroscopic level looks at the structure of matter that gives rise to these interactions. At O Level, students have been i ...
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The submicroscopic level looks at the structure of matter that gives rise to these interactions. At O Level, students have been i ...
CHAPTER
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
chapter 21 chemistry of the main-group elements i
... bond them together. To bond these four atoms into a chain requires three electron pairs. Since each electron pair in a bridging bond replaces two “normal” bonds, there must be at least two bridging bonds in the B4 H10 molecules. By analogy with B2 H 6 , we might write the structure below left. But t ...
... bond them together. To bond these four atoms into a chain requires three electron pairs. Since each electron pair in a bridging bond replaces two “normal” bonds, there must be at least two bridging bonds in the B4 H10 molecules. By analogy with B2 H 6 , we might write the structure below left. But t ...
The Bio-Organometallic Chemistry of Technetium and Rhenium
... refers to a group of atoms that have the same number of electrons as each other and similar connectivity of atoms. The formal oxidation state is defined as the hypothetical charge that an atom would have if all bonds to atoms of different elements were completely ionic. The oxidation state does not ...
... refers to a group of atoms that have the same number of electrons as each other and similar connectivity of atoms. The formal oxidation state is defined as the hypothetical charge that an atom would have if all bonds to atoms of different elements were completely ionic. The oxidation state does not ...
File
... 3. __________ classified the then known elements into metals, non metals and their derivatives. (Dobreiner, Al-Razi, Newlands, Mendeleeve) 4. In 1817, a German chemist, __________ made use of the idea of relationship between atomic weights and properties of elements for the classification of element ...
... 3. __________ classified the then known elements into metals, non metals and their derivatives. (Dobreiner, Al-Razi, Newlands, Mendeleeve) 4. In 1817, a German chemist, __________ made use of the idea of relationship between atomic weights and properties of elements for the classification of element ...
Learning Outcomes
... (f) define relative atomic mass, Ar .............................................................................................. 18 (g) define relative molecular mass, Mr, and calculate relative molecular mass (and relative formula mass) as the sum of relative atomic masses........................ ...
... (f) define relative atomic mass, Ar .............................................................................................. 18 (g) define relative molecular mass, Mr, and calculate relative molecular mass (and relative formula mass) as the sum of relative atomic masses........................ ...
ordinary level chemistry syllabus
... not have been successful without the participation of a range of different education stakeholders and the financial support from different donors. For this I would like to express my deep gratitude. My thanks firstly goes to the Rwanda Education leadership who supervised the curriculum review proces ...
... not have been successful without the participation of a range of different education stakeholders and the financial support from different donors. For this I would like to express my deep gratitude. My thanks firstly goes to the Rwanda Education leadership who supervised the curriculum review proces ...
Redox Reactions C12-1-10
... teacher) for station #11. Number them from 1-13. Do not empty your test tubes when you leave the station. Follow the instructions for each station. Describe briefly your observations for every reaction and write balanced chemical equations. Look for: ...
... teacher) for station #11. Number them from 1-13. Do not empty your test tubes when you leave the station. Follow the instructions for each station. Describe briefly your observations for every reaction and write balanced chemical equations. Look for: ...
Test-tube Reactions - University of Manitoba
... teacher) for station #11. Number them from 1-13. Do not empty your test tubes when you leave the station. Follow the instructions for each station. Describe briefly your observations for every reaction and write balanced chemical equations. Look for: ...
... teacher) for station #11. Number them from 1-13. Do not empty your test tubes when you leave the station. Follow the instructions for each station. Describe briefly your observations for every reaction and write balanced chemical equations. Look for: ...
Nickel(II) cis- and trans-Dimethyl Complexes of
... been prepared and characterized by single-crystal X-ray diffraction, and the complex [Ni(tBuCCmeth)Me2] (3) has been observed spectroscopically. Thermal decomposition studies show that 2 eliminates methane rapidly and quantitatively above 50 °C by a predominantly unimolecular dissociative pathway wi ...
... been prepared and characterized by single-crystal X-ray diffraction, and the complex [Ni(tBuCCmeth)Me2] (3) has been observed spectroscopically. Thermal decomposition studies show that 2 eliminates methane rapidly and quantitatively above 50 °C by a predominantly unimolecular dissociative pathway wi ...
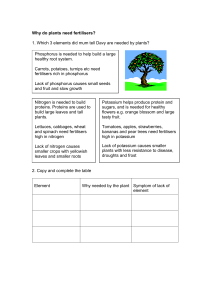
Fertilisers
... Nitric acid can be made from nitrogen dioxide but, as we have seen it is expensive to make nitrogen dioxide by direct combination of nitrogen and oxygen because of the high temperatures needed. An alternative way to make nitrogen dioxide is by the catalytic oxidation of ammonia using a platinum cata ...
... Nitric acid can be made from nitrogen dioxide but, as we have seen it is expensive to make nitrogen dioxide by direct combination of nitrogen and oxygen because of the high temperatures needed. An alternative way to make nitrogen dioxide is by the catalytic oxidation of ammonia using a platinum cata ...
Chemistry
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
chemistry notes on the mole - lessons
... and hydrogen peroxide H2O2(l). Both of these compounds contain hydrogen and oxygen. The only difference between the two, is that there is one extra oxygen atom in the hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both subs ...
... and hydrogen peroxide H2O2(l). Both of these compounds contain hydrogen and oxygen. The only difference between the two, is that there is one extra oxygen atom in the hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both subs ...
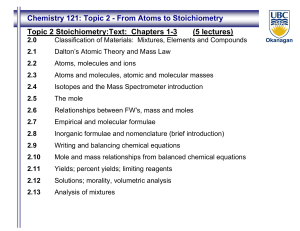
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined ...
... ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined ...
formula writing and nomenclature of inorganic - Parkway C-2
... The two S atoms in the compound must contribute a total charge of +10 in order for the net charge of CaS2O6 to be zero. Therefore, each S atom must have an oxidation number of +5. 3. Determine the oxidation number of Cu in CuSO4. Cu is an element that can have more than one oxidation state (see Tabl ...
... The two S atoms in the compound must contribute a total charge of +10 in order for the net charge of CaS2O6 to be zero. Therefore, each S atom must have an oxidation number of +5. 3. Determine the oxidation number of Cu in CuSO4. Cu is an element that can have more than one oxidation state (see Tabl ...
lecture ch1-3 chem161pikul
... In chemical reactions, atoms rearrange but do not break apart. 3. In any sample of a pure element, all atoms are identical in mass and other properties. 4. Atoms of different elements differ in mass and other properties. 5. In a given compound, constituent atoms are always present in same fixed n ...
... In chemical reactions, atoms rearrange but do not break apart. 3. In any sample of a pure element, all atoms are identical in mass and other properties. 4. Atoms of different elements differ in mass and other properties. 5. In a given compound, constituent atoms are always present in same fixed n ...
11.2 Types of Chemical Reactions
... Have students research the meaning of synthesis. Have them investigate the synthesis of ...
... Have students research the meaning of synthesis. Have them investigate the synthesis of ...
9647 H2 Chemistry
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
... Candidates will be assumed to have knowledge and understanding of Chemistry at O level, as a single subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts a ...
The mole and calculations
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
Chemistry Transition Information
... Atoms and molecules are very small – far too small to count individually! It is important to know how much of something we have, but we count particles in MOLES because you get simpler numbers ...
... Atoms and molecules are very small – far too small to count individually! It is important to know how much of something we have, but we count particles in MOLES because you get simpler numbers ...
Analytical Techniques for Elemental Analysis of Minerals
... performed at the micrometer scale are preferred. Even when minerals have larger sizes so that sample amounts of 50 mg or even grams are available, there is another problem to consider: such “bulk samples” may contain abundant inclusions of other minerals, which can result in a systematic error in mi ...
... performed at the micrometer scale are preferred. Even when minerals have larger sizes so that sample amounts of 50 mg or even grams are available, there is another problem to consider: such “bulk samples” may contain abundant inclusions of other minerals, which can result in a systematic error in mi ...
(+1) + - Edublogs
... For electrons that are shared in these compounds, we assign the shared electrons to the most electronegative element. We are just acting as though the electronegativity difference was large enough for the transfer of electrons to occur. ...
... For electrons that are shared in these compounds, we assign the shared electrons to the most electronegative element. We are just acting as though the electronegativity difference was large enough for the transfer of electrons to occur. ...