File
... Alkali metals have a ns1 valence shell electron configuration. Alkali metals lose this valence electron with relative ease to form M+ cations when in ionic compounds. They all are easily oxidized. Therefore, in order to prepare the pure metals, alkali metals must be produced in the absence of materi ...
... Alkali metals have a ns1 valence shell electron configuration. Alkali metals lose this valence electron with relative ease to form M+ cations when in ionic compounds. They all are easily oxidized. Therefore, in order to prepare the pure metals, alkali metals must be produced in the absence of materi ...
The d-Block Elements
... next element—Ce—is 6s25d04f2. From this point through element 71, added electrons enter the 4f subshell, giving rise to the 14 elements known as the lanthanides. After the 4f subshell is filled, the 5d subshell is populated, producing the third row of the transition metals. Next comes the seventh pe ...
... next element—Ce—is 6s25d04f2. From this point through element 71, added electrons enter the 4f subshell, giving rise to the 14 elements known as the lanthanides. After the 4f subshell is filled, the 5d subshell is populated, producing the third row of the transition metals. Next comes the seventh pe ...
2.6 M - Thierry Karsenti
... This module in organic chemistry is designed to prepare students that wish to become teachers : Student teachers must have knowledge of the key concepts and classification tools of organic chemistry that include functional groups of hydrocarbons,alcohols and ethers,aldehydes and ketones,alkyl halide ...
... This module in organic chemistry is designed to prepare students that wish to become teachers : Student teachers must have knowledge of the key concepts and classification tools of organic chemistry that include functional groups of hydrocarbons,alcohols and ethers,aldehydes and ketones,alkyl halide ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Chemistry 101L
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
Recycling and Chemical Mathematics
... release oxygen gas in the process. The animal life of Biosphere 2, through the process of breathing (respiration), takes in atmospheric oxygen and releases carbon dioxide. If everything could be arranged to come out even, a stable atmosphere with desirable levels of oxygen and carbon dioxide would b ...
... release oxygen gas in the process. The animal life of Biosphere 2, through the process of breathing (respiration), takes in atmospheric oxygen and releases carbon dioxide. If everything could be arranged to come out even, a stable atmosphere with desirable levels of oxygen and carbon dioxide would b ...
Metalloid Al- and Ga-clusters: a novel dimension in organometallic
... structure analysis.4 The largest cluster of this type is a Pd145 cluster for which—although only two crystals have been obtained so far—the structure could be solved by L. F. Dahl et al.:5 There is a centre of 55 naked Pd atoms surrounded by 90 ligand-bearing Pd atoms. The many synthetic results for ...
... structure analysis.4 The largest cluster of this type is a Pd145 cluster for which—although only two crystals have been obtained so far—the structure could be solved by L. F. Dahl et al.:5 There is a centre of 55 naked Pd atoms surrounded by 90 ligand-bearing Pd atoms. The many synthetic results for ...
Holt Modern Chemistry Workbook
... A molecular compound is any chemical compound whose simplest units are molecules. In other words, a single molecule of any molecular compound is an individual unit that is capable of existing on its own. A molecule may contain two or more atoms of the same element, as in oxygen. Or, a molecule may c ...
... A molecular compound is any chemical compound whose simplest units are molecules. In other words, a single molecule of any molecular compound is an individual unit that is capable of existing on its own. A molecule may contain two or more atoms of the same element, as in oxygen. Or, a molecule may c ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
Chemistry
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The submicroscopic level looks at the structure of matter that gives rise to these interactions. At O-Level, students have been i ...
... Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The submicroscopic level looks at the structure of matter that gives rise to these interactions. At O-Level, students have been i ...
+ (aq)
... delocalizing into the electron sea. The strength of metallic bond in these metals is thus very strong. In the case of s-block metals, the metallic radius is larger and most of them do not have close-packed structures. Also , as they have only one or two valence electrons per atom delocalizing into t ...
... delocalizing into the electron sea. The strength of metallic bond in these metals is thus very strong. In the case of s-block metals, the metallic radius is larger and most of them do not have close-packed structures. Also , as they have only one or two valence electrons per atom delocalizing into t ...
Section 3.5 Ionic Compounds: Formulas and Names
... Molecular Compounds: Formulas and Names Solution • The compound NCl3 is nitrogen trichloride , but AlCl3 is just aluminum chloride. Why? • NCl3 is a covalent (molecular compound). Since nitrogen and chlorine can combine more than one way it is necessary to indicate the number of chlorines. • AlCl3 i ...
... Molecular Compounds: Formulas and Names Solution • The compound NCl3 is nitrogen trichloride , but AlCl3 is just aluminum chloride. Why? • NCl3 is a covalent (molecular compound). Since nitrogen and chlorine can combine more than one way it is necessary to indicate the number of chlorines. • AlCl3 i ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
2014_S4_CHM_NORMAL (ALL)
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
Topic 1 - Coral Gables Senior High
... Antoine-Laurent Lavoisier (1743–1794) is often called the ‘father of chemistry’. His many accomplishments include the naming of oxygen and hydrogen, the early development of the metric system, and a standardization of chemical nomenclature. Most importantly, he established an understanding of combus ...
... Antoine-Laurent Lavoisier (1743–1794) is often called the ‘father of chemistry’. His many accomplishments include the naming of oxygen and hydrogen, the early development of the metric system, and a standardization of chemical nomenclature. Most importantly, he established an understanding of combus ...
Stoichiometric relationships
... Antoine-Laurent Lavoisier (1743–1794) is often called the ‘father of chemistry’. His many accomplishments include the naming of oxygen and hydrogen, the early development of the metric system, and a standardization of chemical nomenclature. Most importantly, he established an understanding of combus ...
... Antoine-Laurent Lavoisier (1743–1794) is often called the ‘father of chemistry’. His many accomplishments include the naming of oxygen and hydrogen, the early development of the metric system, and a standardization of chemical nomenclature. Most importantly, he established an understanding of combus ...
A Dictionary of the New Chymical Nomenclature
... Sulphureous acid Volatile sulphureous acid Phlogisticated vitriolic acid Spirit of sulphur ...
... Sulphureous acid Volatile sulphureous acid Phlogisticated vitriolic acid Spirit of sulphur ...
Slide 1
... 1. What are the reactants in this chemical equation? 2. What are the products in this chemical equation? 3. Are there the same number of atoms on both sides of the equation? a. Were any atoms destroyed or created? b. Was the Law of Conservation of Matter maintained? ...
... 1. What are the reactants in this chemical equation? 2. What are the products in this chemical equation? 3. Are there the same number of atoms on both sides of the equation? a. Were any atoms destroyed or created? b. Was the Law of Conservation of Matter maintained? ...
Chapter 4 – Part 1
... Know how to find the number of protons and neutrons in an isotope Know the 3 isotopes of hydrogen Know how to calculate the average atomic mass from Isotopic Abundances Know how to calculate Isotopic Abundances Know what a mass spectrometer is and what it measures Know the difference between a group ...
... Know how to find the number of protons and neutrons in an isotope Know the 3 isotopes of hydrogen Know how to calculate the average atomic mass from Isotopic Abundances Know how to calculate Isotopic Abundances Know what a mass spectrometer is and what it measures Know the difference between a group ...
The Mole
... Pure and Applied Chemistry (IUPAC) defines "mole:" • The mole is the amount of substance of a system that contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electr ...
... Pure and Applied Chemistry (IUPAC) defines "mole:" • The mole is the amount of substance of a system that contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electr ...
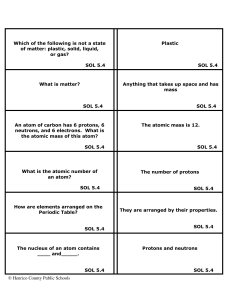
Matter Flashcards 5 - Henrico County Public Schools
... A molecule of an element is two or more atoms of the same kind of element joined together. A molecule of a compound is made of different kinds of elements joined together. SOL 5.4 A mixture is two or more substances joined physically. A compound is two or more substances joined ...
... A molecule of an element is two or more atoms of the same kind of element joined together. A molecule of a compound is made of different kinds of elements joined together. SOL 5.4 A mixture is two or more substances joined physically. A compound is two or more substances joined ...
1411FINALSAMPLE+KEY - Houston Community College
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...