03_Worked_Examples
... will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is to determine the formula weight of glucose: 6 C atoms = 6(12.0 amu) = 72.0 amu 12 H atoms = 12(1.0 amu) = 12.0 amu 6 O atoms = 6(16.0 amu) = 96.0 amu 180.0 amu Because glucose has a formula weight of 180.0 amu ...
... will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is to determine the formula weight of glucose: 6 C atoms = 6(12.0 amu) = 72.0 amu 12 H atoms = 12(1.0 amu) = 12.0 amu 6 O atoms = 6(16.0 amu) = 96.0 amu 180.0 amu Because glucose has a formula weight of 180.0 amu ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de-Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de-Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
GCE Getting Started - Edexcel
... From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they had been taught or, if they are from different centres, u ...
... From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they had been taught or, if they are from different centres, u ...
Syllabus and Regulations for 2-year, 4
... by the College authority and will be duly notified. Total duration of the course is two years [hereafter, “Course” refers to M. Sc. Course in Chemistry and “Paper” refers to the individual papers of 75/80/85/90/100 marks divided into two halves: Group-A: Theoretical (50-marks) and Group-B: Practical ...
... by the College authority and will be duly notified. Total duration of the course is two years [hereafter, “Course” refers to M. Sc. Course in Chemistry and “Paper” refers to the individual papers of 75/80/85/90/100 marks divided into two halves: Group-A: Theoretical (50-marks) and Group-B: Practical ...
Organic and Bio-Molecular Chemistry
... study of these compounds, defined Natural Compounds, as far as structure, properties and biological role is concerned, is the subject matter of Organic and Bio-Molecular Chemistry. Chemists have been able to synthesize a great variety of new compounds with a skeleton mainly based on carbon atoms; th ...
... study of these compounds, defined Natural Compounds, as far as structure, properties and biological role is concerned, is the subject matter of Organic and Bio-Molecular Chemistry. Chemists have been able to synthesize a great variety of new compounds with a skeleton mainly based on carbon atoms; th ...
materials required/recommended for this paper
... Spare pages are included at the end of this booklet. They can be used for planning your responses and/or as additional space if required to continue an answer. Planning: If you use the spare pages for planning, indicate this clearly at the top of the page. Continuing an answer: If you need to us ...
... Spare pages are included at the end of this booklet. They can be used for planning your responses and/or as additional space if required to continue an answer. Planning: If you use the spare pages for planning, indicate this clearly at the top of the page. Continuing an answer: If you need to us ...
Deuterium fractionation of methylamine through atomic grain
... Institute of Low Temperature Science, Hokkaido University, Japan Interstellar methylamine (CH3NH2) was first found in 1974 toward Sgr B2 and Ori A [1]. This finding is of interest in view of astrobiology because methylamine could be a precursor of amino acid in space [2]. Laboratory studies revealed ...
... Institute of Low Temperature Science, Hokkaido University, Japan Interstellar methylamine (CH3NH2) was first found in 1974 toward Sgr B2 and Ori A [1]. This finding is of interest in view of astrobiology because methylamine could be a precursor of amino acid in space [2]. Laboratory studies revealed ...
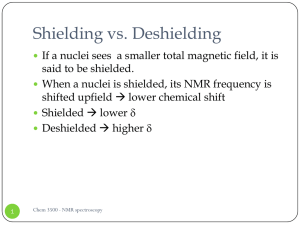
Shielding vs. Deshielding
... on the allylic carbon atoms by shifting their chemical shift up (deshielding) ...
... on the allylic carbon atoms by shifting their chemical shift up (deshielding) ...
chem - CBSE Guess
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
STUDY GUIDE
... have a ring structure and bonding that causes them to be chemically stable. Measurements show that the bonds in a benzene ring are all equal in length. 1. Is the following statement true or false? If you think the statement is false, rewrite it to make it true: The commercial use of benzene has ...
... have a ring structure and bonding that causes them to be chemically stable. Measurements show that the bonds in a benzene ring are all equal in length. 1. Is the following statement true or false? If you think the statement is false, rewrite it to make it true: The commercial use of benzene has ...
DOE Chemistry 1
... subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology, Prints, and Drawings; and Nuclear Physics and Reactor Theory. The handbooks ...
... subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology, Prints, and Drawings; and Nuclear Physics and Reactor Theory. The handbooks ...
Chapter12
... numbers of molecules are not possible to count, so we use the mol = 6.02 X 1023 molecules instead c. Moles - The coefficients in a balanced chemical equation tells us the number of moles of reactants and products. The equation tells us that 1 mol of N2(g) reacts with 3mol of H2(g) to yield 2 mol of ...
... numbers of molecules are not possible to count, so we use the mol = 6.02 X 1023 molecules instead c. Moles - The coefficients in a balanced chemical equation tells us the number of moles of reactants and products. The equation tells us that 1 mol of N2(g) reacts with 3mol of H2(g) to yield 2 mol of ...
Chapter 3 – Atomic Structure and Properties
... Note that the atomic radii of H and Rb are 0.37 Å and 2.11 Å, respectively. ...
... Note that the atomic radii of H and Rb are 0.37 Å and 2.11 Å, respectively. ...
Experiment 9
... b) don’t keep the gas tap open while waiting to the lighter; c) close the gate valve at the end of the laboratory session; d) if you smell the gas while the tap is closed, inform the ...
... b) don’t keep the gas tap open while waiting to the lighter; c) close the gate valve at the end of the laboratory session; d) if you smell the gas while the tap is closed, inform the ...
Redox
... the loss/gain of hydrogen. Oxidation is the gain of oxygen or the loss of hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
... the loss/gain of hydrogen. Oxidation is the gain of oxygen or the loss of hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
Chemistry Challenge Problems
... for these elements today are very different from their accepted atomic masses at the time Döbereiner made his observations. Döbereiner also observed that strontium, calcium, and barium showed a gradual gradation in their properties, with the values of some of strontium’s properties being about midwa ...
... for these elements today are very different from their accepted atomic masses at the time Döbereiner made his observations. Döbereiner also observed that strontium, calcium, and barium showed a gradual gradation in their properties, with the values of some of strontium’s properties being about midwa ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.