Unit 1
... Students might discuss the fundamentals of collision theory using potential energy diagrams and energy considerations. Controlling reaction rates is important in many commercial and industrial processes. By applying collision theory to the rates of fast and slow reactions, teachers might look for co ...
... Students might discuss the fundamentals of collision theory using potential energy diagrams and energy considerations. Controlling reaction rates is important in many commercial and industrial processes. By applying collision theory to the rates of fast and slow reactions, teachers might look for co ...
Safer by Design - Environment America
... for insightful review and helpful suggestions on drafts of this report. Additional thanks go to the many businesses and individuals who provided information. Finally, thanks to Tony Dutzik at Frontier Group and to Carolyn Kramer and Laura Deetz for research and editorial support. Environment America ...
... for insightful review and helpful suggestions on drafts of this report. Additional thanks go to the many businesses and individuals who provided information. Finally, thanks to Tony Dutzik at Frontier Group and to Carolyn Kramer and Laura Deetz for research and editorial support. Environment America ...
Calculations on the equations reaction
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
CHAPTER 3 STOICHIOMETRY:
... Yes; because there are equal numbers of both N and O atoms in the two boxes ...
... Yes; because there are equal numbers of both N and O atoms in the two boxes ...
Burning a Candle in a Vessel, a Simple Experiment
... analysis for the atmosphere of the experiment of burning a piece of paper (Vitz 2000), shows a consumption of a 56% of the initial amount of oxygen. Although the chemical reaction for the candle experiment is different, the previous GC result shows that the amount of oxygen that reacts in the burnin ...
... analysis for the atmosphere of the experiment of burning a piece of paper (Vitz 2000), shows a consumption of a 56% of the initial amount of oxygen. Although the chemical reaction for the candle experiment is different, the previous GC result shows that the amount of oxygen that reacts in the burnin ...
Chemistry - WorkNotes
... Recognize the issues of statistical variability and the need for controlled tests. ...
... Recognize the issues of statistical variability and the need for controlled tests. ...
Significant Figures
... Zeros at the end of a number and to the right of a decimal are significant, for example: * 35.00 has four significant figures * 8,000.000000 has ten significant figures Zeros at the end of a number without a decimal point may or may not be significant, and are therefore ambiguous, for example: * 1,0 ...
... Zeros at the end of a number and to the right of a decimal are significant, for example: * 35.00 has four significant figures * 8,000.000000 has ten significant figures Zeros at the end of a number without a decimal point may or may not be significant, and are therefore ambiguous, for example: * 1,0 ...
NUCL 1 Early life of Albert Ghiorso: Preparation for future role as
... Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States Although young Albert was a good student in classical subjects, he early on showed his mechanical ability. His family lived close to the Oakland Airport and Albert set his sights on becoming an aeronautical engineer. When he gr ...
... Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States Although young Albert was a good student in classical subjects, he early on showed his mechanical ability. His family lived close to the Oakland Airport and Albert set his sights on becoming an aeronautical engineer. When he gr ...
Stoichiometry of Chemical Reactions
... System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vig ...
... System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vig ...
Stoichiometry of Chemical Reactions
... System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vig ...
... System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vig ...
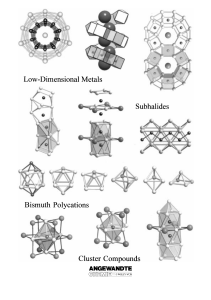
From the Metal to the Molecule
... metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which cont ...
... metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which cont ...
Personal Tutor - Macmillan Learning
... examination would not truly test your understanding of concepts and your ability to solve problems if the professor merely put on the problems you had already seen. If you use your textbook, the Personal Tutor, and any other sources available you can be successful in this chemistry course. ...
... examination would not truly test your understanding of concepts and your ability to solve problems if the professor merely put on the problems you had already seen. If you use your textbook, the Personal Tutor, and any other sources available you can be successful in this chemistry course. ...
ANSWERS Problem Set 5a – Chemical Reactions
... 1) Define a chemical reaction. Use the terms “reactant and product” and write at least three sentences in your definition. Don’t give examples… just a definition. A chemical reaction is a change of bonds in a substance or between substances. The bonds can be broken, rearranged or new bonds can be fo ...
... 1) Define a chemical reaction. Use the terms “reactant and product” and write at least three sentences in your definition. Don’t give examples… just a definition. A chemical reaction is a change of bonds in a substance or between substances. The bonds can be broken, rearranged or new bonds can be fo ...
Task 4 6 points - Austrian Chemistry Olympiad
... for this part of the task in total, wrong answers will lead to deduction of points.) ...
... for this part of the task in total, wrong answers will lead to deduction of points.) ...
s_block - ilc.edu.hk
... Chemical formulae of some Group II compounds and the oxidation states of Group II elements in the compounds Group II element ...
... Chemical formulae of some Group II compounds and the oxidation states of Group II elements in the compounds Group II element ...
CHemiStrY - Cabrillo College
... A chemistry major usually transfers to a four-year institution to complete a bachelor’s degree. Many also go on to earn Masters or Ph.D.s, since advanced degrees generally lead to more rewarding careers. Cabrillo’s chemistry program is articulated with those of the UC and CSU systems and includes th ...
... A chemistry major usually transfers to a four-year institution to complete a bachelor’s degree. Many also go on to earn Masters or Ph.D.s, since advanced degrees generally lead to more rewarding careers. Cabrillo’s chemistry program is articulated with those of the UC and CSU systems and includes th ...
Organic Molecules
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
2010 Released SOL
... d. the pressure on the water is insufficient to hold the hydrogen and oxygen atoms together, resulting in a decomposition reaction file:///F:/SOL%20Review%20Topics/Chemistry%202010%20SOL/2010_released_sol.htm ...
... d. the pressure on the water is insufficient to hold the hydrogen and oxygen atoms together, resulting in a decomposition reaction file:///F:/SOL%20Review%20Topics/Chemistry%202010%20SOL/2010_released_sol.htm ...
Photoprocesses in protoplanetary disks
... oscillator strengths to the A2Pu and 22Pu states—the only dipole allowed states below 13.6 eV and above the dissociation limit—are only f = 0.005 each. CO+. CO+ is strongly bound with a dissociation energy of 8.34 eV, but its higher excited D2P, G, E and F 2S+ states are likely (pre-)dissociative.38 ...
... oscillator strengths to the A2Pu and 22Pu states—the only dipole allowed states below 13.6 eV and above the dissociation limit—are only f = 0.005 each. CO+. CO+ is strongly bound with a dissociation energy of 8.34 eV, but its higher excited D2P, G, E and F 2S+ states are likely (pre-)dissociative.38 ...
department of pure and applied chemistry
... skimming and scanning; and use of library, including cataloguing systems, locating books and journals, lending/borrowing, reference materials, indexing and examination taking techniques as well as book review. GES 1012: English and Communication Skills II (2 credit units) This is a continuation of G ...
... skimming and scanning; and use of library, including cataloguing systems, locating books and journals, lending/borrowing, reference materials, indexing and examination taking techniques as well as book review. GES 1012: English and Communication Skills II (2 credit units) This is a continuation of G ...
3.0 Properties of Phosgene
... start reacting with steel, weakening the piping and vessels. At 483oF, chlorine will ignite iron and produce a fire. Detection of these impurity generated reactions can be noticed by a rapid rise in the temperature of the feed gas after the carbon monoxide and chlorine mixing point. The use of high ...
... start reacting with steel, weakening the piping and vessels. At 483oF, chlorine will ignite iron and produce a fire. Detection of these impurity generated reactions can be noticed by a rapid rise in the temperature of the feed gas after the carbon monoxide and chlorine mixing point. The use of high ...
Chem 107 - Hughbanks Exam 1
... (5) (7 points) The following full sets of potential compounds are made up of common cations and anions. Which full sets are most likely to actually exist (all 3 compounds in a set must be sensible to qualify)? There may be more than one correct choice; enter ALL the correct letters, in alphabetical ...
... (5) (7 points) The following full sets of potential compounds are made up of common cations and anions. Which full sets are most likely to actually exist (all 3 compounds in a set must be sensible to qualify)? There may be more than one correct choice; enter ALL the correct letters, in alphabetical ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.