Boronic acids facilitate rapid oxime condensations at neutral pH
... 2 & 8) but the hydrolysed boronic acid 2a was the main product with only 10% of pinacol ester oxime 2f observed; aer ninety minutes only 2a was present. The ketoximine pinacol product (entry 9), on the other hand, was more stable and comprised 97% of the product aer the rst injection (the remaind ...
... 2 & 8) but the hydrolysed boronic acid 2a was the main product with only 10% of pinacol ester oxime 2f observed; aer ninety minutes only 2a was present. The ketoximine pinacol product (entry 9), on the other hand, was more stable and comprised 97% of the product aer the rst injection (the remaind ...
Exam Edge Digital
... in covalently-bonded molecules (showing only the electrons in the outside shells of the atoms). You should be familiar with how overlap of atomic orbitals is used to explain the formation of covalent bonds and be able to distinguish between sigma and pi bonding. You should also know how the number o ...
... in covalently-bonded molecules (showing only the electrons in the outside shells of the atoms). You should be familiar with how overlap of atomic orbitals is used to explain the formation of covalent bonds and be able to distinguish between sigma and pi bonding. You should also know how the number o ...
Isomers and Isomerism Isomers
... Isomers are different compounds with the same molecular formula. They have the same numbers of same kinds of atoms, but the differ in the way that those atoms are attached to one another, or how they are oriented in space,(Greek: isos= equal; meros=part) Isomerism The presence of two or more compo ...
... Isomers are different compounds with the same molecular formula. They have the same numbers of same kinds of atoms, but the differ in the way that those atoms are attached to one another, or how they are oriented in space,(Greek: isos= equal; meros=part) Isomerism The presence of two or more compo ...
Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic
... • Chemical Reactions do not always go the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
... • Chemical Reactions do not always go the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... Electrodes can be placed in the reaction mixture and the increase/decrease in conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formati ...
... Electrodes can be placed in the reaction mixture and the increase/decrease in conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formati ...
Integrated Curriculum for Secondary Schools CHEMISTRY Form 4
... depends on science and technology. It is envisaged that success in providing quality science education to Malaysians from an early age will serve to spearhead the nation into becoming a knowledge society and a competitive player in the global arena. Towards this end, the Malaysian education system i ...
... depends on science and technology. It is envisaged that success in providing quality science education to Malaysians from an early age will serve to spearhead the nation into becoming a knowledge society and a competitive player in the global arena. Towards this end, the Malaysian education system i ...
4 Expressing and Measuring Chemical Change
... Historical evidence connects Joseph Priestley’s experiments with oxygen with another famous chemist, Antoine Lavoisier. Lavoisier’s experiments were more quantitative than those of Priestley. That is, Lavoisier liked to measure the volumes and masses of the chemicals he studied. Lavoisier is general ...
... Historical evidence connects Joseph Priestley’s experiments with oxygen with another famous chemist, Antoine Lavoisier. Lavoisier’s experiments were more quantitative than those of Priestley. That is, Lavoisier liked to measure the volumes and masses of the chemicals he studied. Lavoisier is general ...
AVOGADRO EXAMS 1991 - 2002 PRACTICE BOOKLET
... (a) reaction of acids with carbonates (c) complete combustion of methane (e) action of steam on hot coke ...
... (a) reaction of acids with carbonates (c) complete combustion of methane (e) action of steam on hot coke ...
The Mole
... Pure and Applied Chemistry (IUPAC) defines "mole:" • The mole is the amount of substance of a system that contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electr ...
... Pure and Applied Chemistry (IUPAC) defines "mole:" • The mole is the amount of substance of a system that contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electr ...
BASIC CONCEPTS OF CHEMISTRY
... process in time. This approach is called the thermodynamics. For convenience, the objects of study should be isolated. Such a collection of bodies extracted from the space, is called the system . If no mass and heat transfer exists between the system and the surrounding environment, the system is ca ...
... process in time. This approach is called the thermodynamics. For convenience, the objects of study should be isolated. Such a collection of bodies extracted from the space, is called the system . If no mass and heat transfer exists between the system and the surrounding environment, the system is ca ...
Chapter 3
... → are the products. In all chemical reactions some number may occurs in front of the reactant and the products (if the number is 1 we ignore it as it its understandable without being written), these numbers indicates the number of molecules (mol) that reacted or produced. These numbers are very impo ...
... → are the products. In all chemical reactions some number may occurs in front of the reactant and the products (if the number is 1 we ignore it as it its understandable without being written), these numbers indicates the number of molecules (mol) that reacted or produced. These numbers are very impo ...
Document
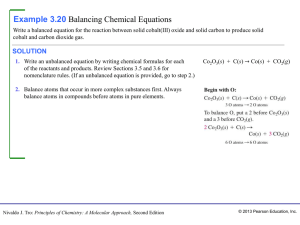
... incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical experiments depends.” --Antoine Lavoisier, 1789 Chemistry: The Central Science, Elev ...
... incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical experiments depends.” --Antoine Lavoisier, 1789 Chemistry: The Central Science, Elev ...
CHE 110 Dr. Nicholas Bizier Office DS 337b email
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
406 K (English version)
... Course objective This is the first part of a first-year university course in Chemistry which aims at preparing students wishing to become teachers. It outlines basic concepts and tools in chemistry which include matter and measurement, the structure of the atom, molecules and compounds as well as ch ...
... Course objective This is the first part of a first-year university course in Chemistry which aims at preparing students wishing to become teachers. It outlines basic concepts and tools in chemistry which include matter and measurement, the structure of the atom, molecules and compounds as well as ch ...
M.Sc. Chemistry (Two year Course)
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
Document
... CHECK The units of the final answer are correct. The magnitude of the final answer (38 kg H 2SO4) is larger than the amount of SO2 given (25 kg). This is reasonable because in the reaction each SO 2 molecule “gains weight” by reacting with O2 and H2O. For Practice 4.2 Another component of acid rain ...
... CHECK The units of the final answer are correct. The magnitude of the final answer (38 kg H 2SO4) is larger than the amount of SO2 given (25 kg). This is reasonable because in the reaction each SO 2 molecule “gains weight” by reacting with O2 and H2O. For Practice 4.2 Another component of acid rain ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... In your previous classes, you have studied how to write chemical formula of a sustance. For example, water is represented by H2O, carbon dioxide is represented by CO2, methane is represented by CH4, dinitrogen penta oxide is represented by N2O5, and so on. You are aware, formula for a molecule uses ...
... In your previous classes, you have studied how to write chemical formula of a sustance. For example, water is represented by H2O, carbon dioxide is represented by CO2, methane is represented by CH4, dinitrogen penta oxide is represented by N2O5, and so on. You are aware, formula for a molecule uses ...
Regents Chemistry - New York Science Teacher
... • The balanced equation below represents a molecule of bromine separating into two bromine atoms. Br2 -> Br + Br What occurs during the change? ...
... • The balanced equation below represents a molecule of bromine separating into two bromine atoms. Br2 -> Br + Br What occurs during the change? ...
Chemistry - Bulletin < Brown
... of the concentration program Biochemistry and Molecular Biology. In earlier years of this discipline, the focus was on structure and function of proteins, nucleic acids, lipids, carbohydrates and small molecules such as vitamins. Today the logical approach and tools of biochemical science are being ...
... of the concentration program Biochemistry and Molecular Biology. In earlier years of this discipline, the focus was on structure and function of proteins, nucleic acids, lipids, carbohydrates and small molecules such as vitamins. Today the logical approach and tools of biochemical science are being ...
Syllabus Cambridge IGCSE Chemistry (US) Syllabus Code 0439 For examination in 2013
... Cambridge ICE is the group award of the IGCSE. It requires the study of subjects drawn from the five different IGCSE subject groups. It gives Centers the opportunity to benefit from offering a broad and balanced curriculum by recognizing the achievements of students who pass examinations in at least ...
... Cambridge ICE is the group award of the IGCSE. It requires the study of subjects drawn from the five different IGCSE subject groups. It gives Centers the opportunity to benefit from offering a broad and balanced curriculum by recognizing the achievements of students who pass examinations in at least ...
GCE Getting Started - Edexcel
... Know that electronegativity is the ability of an atom to attract the bonding electrons in a covalent bond. Know that ionic and covalent bonding are the extremes of a continuum of bonding type and that electronegativity differences lead to bond polarity in bonds and molecules. Understand the differen ...
... Know that electronegativity is the ability of an atom to attract the bonding electrons in a covalent bond. Know that ionic and covalent bonding are the extremes of a continuum of bonding type and that electronegativity differences lead to bond polarity in bonds and molecules. Understand the differen ...
Chemistry Essentials For Dummies
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
redox reaction - Seattle Central College
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
CHEMICAL EQUATIONS - Clayton State University
... Consider the following Sodium (Na) has an atomic mass of 22.99 u This implies that the mass of 1 mole of Na = 22.99 g Molar mass of Na = 22.99 g/mol Formula mass of NaCl = 58.44 u The mass of 1 mole of NaCl = 58.44 g Molar mass of NaCl = 58.88 g/mol Formula mass of CaCO3 = 100.09 u The mass of 1 mol ...
... Consider the following Sodium (Na) has an atomic mass of 22.99 u This implies that the mass of 1 mole of Na = 22.99 g Molar mass of Na = 22.99 g/mol Formula mass of NaCl = 58.44 u The mass of 1 mole of NaCl = 58.44 g Molar mass of NaCl = 58.88 g/mol Formula mass of CaCO3 = 100.09 u The mass of 1 mol ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.