11 myp covalent bonding
... What about carbon and the two oxygen atoms in carbon dioxide? And, lastly, what about oxygen and the two hydrogen atoms in water? What keeps atoms together in in the molecule of a compound (and also in the molecule of some elements) is an attractive electrostatic (electromagnetic) force, referred to ...
... What about carbon and the two oxygen atoms in carbon dioxide? And, lastly, what about oxygen and the two hydrogen atoms in water? What keeps atoms together in in the molecule of a compound (and also in the molecule of some elements) is an attractive electrostatic (electromagnetic) force, referred to ...
Problem 28. TUNNELING IN CHEMISTRY
... The superposition principle is applicable to quantum systems only and is not valid when applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device wh ...
... The superposition principle is applicable to quantum systems only and is not valid when applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device wh ...
Concept Development Studies in Chemistry
... development studies will enhance your development of critical, analytical thinking, a skill which is most important to success in Science. As a note, these studies are not intended to be historical developments, although the experiments presented are the ones which led to the concepts discussed. Onl ...
... development studies will enhance your development of critical, analytical thinking, a skill which is most important to success in Science. As a note, these studies are not intended to be historical developments, although the experiments presented are the ones which led to the concepts discussed. Onl ...
2 - Gordon State College
... CHECK YOUR NEIGHBOR Carefully examine the following reaction sequence for the catalytic formation of ozone, O3, from molecular oxygen, O2. Which chemical compound is behaving as the catalyst? O2 + 2 NO 2 NO2 2 NO2 2 NO + 2 O 2 O + 2 O 2 2 O3 A. Nitrogen dioxide, NO2 B. Nitrogen monoxide, NO C. ...
... CHECK YOUR NEIGHBOR Carefully examine the following reaction sequence for the catalytic formation of ozone, O3, from molecular oxygen, O2. Which chemical compound is behaving as the catalyst? O2 + 2 NO 2 NO2 2 NO2 2 NO + 2 O 2 O + 2 O 2 2 O3 A. Nitrogen dioxide, NO2 B. Nitrogen monoxide, NO C. ...
GCE Chemistry Specification (From 2015 - WALES ONLY
... specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of all A level specifications that learners must demonstrate a holistic understanding of the links between different areas of content. In practice, this means that some questions set in A2 unit ...
... specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of all A level specifications that learners must demonstrate a holistic understanding of the links between different areas of content. In practice, this means that some questions set in A2 unit ...
EOCT Physical Science Study Guide August 2008
... think about what you are asked to do. Listen carefully to all the directions. Budget your time. Be sure that you allocate an appropriate amount of time to work on each question on the test. Take a quick break if you begin to feel tired. To do this, put your pencil down, relax in your chair, and take ...
... think about what you are asked to do. Listen carefully to all the directions. Budget your time. Be sure that you allocate an appropriate amount of time to work on each question on the test. Take a quick break if you begin to feel tired. To do this, put your pencil down, relax in your chair, and take ...
File
... When the element % composition is known: 1. Assume 100g sample and change element percents to grams 2. Convert each to moles by dividing by molar mass of each atom ...
... When the element % composition is known: 1. Assume 100g sample and change element percents to grams 2. Convert each to moles by dividing by molar mass of each atom ...
CHEMISTRY 2202
... purple iodine crystals, I2 (s) to the same test tube. The test tube is shaken and allowed to settle for a second time. Based on the results you see below, how can you be certain the fluid in the bottom layer is CCl4(l)? Explain. ...
... purple iodine crystals, I2 (s) to the same test tube. The test tube is shaken and allowed to settle for a second time. Based on the results you see below, how can you be certain the fluid in the bottom layer is CCl4(l)? Explain. ...
PC_Chemistry_Macomb_April08
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
Chemical Bonding and Molecular Geometry
... the electronic structure of the noble gas that precedes it in the periodic table. For groups 1 (the alkali metals) and 2 (the alkaline earth metals), the group numbers are equal to the numbers of valence shell electrons and, consequently, to the charges of the cations formed from atoms of these elem ...
... the electronic structure of the noble gas that precedes it in the periodic table. For groups 1 (the alkali metals) and 2 (the alkaline earth metals), the group numbers are equal to the numbers of valence shell electrons and, consequently, to the charges of the cations formed from atoms of these elem ...
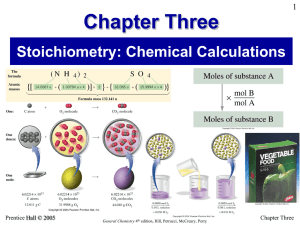
Chapter Three
... There is a phosphorus content of 4.433-g. What is the empirical formula of this compound? • ANS = P2O5 ...
... There is a phosphorus content of 4.433-g. What is the empirical formula of this compound? • ANS = P2O5 ...
29th INTERNATIONAL CHEMISTRY OLYMPIAD PREPARATORY
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
IChO_Comp_Prob_Answ 1997
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
The Effect of Using Computer Simulations in Teaching Chemical
... which may greatly facilitate the mastery of difficult concepts; a case in point is the relation between distance, motion and time (Mintz 1993). Moreover, educators have positive attitudes towards using computers to improve their teaching pedagogy (Govender and Maistry 2012). It is also reported that ...
... which may greatly facilitate the mastery of difficult concepts; a case in point is the relation between distance, motion and time (Mintz 1993). Moreover, educators have positive attitudes towards using computers to improve their teaching pedagogy (Govender and Maistry 2012). It is also reported that ...
EDEXCEL A LeveL - Hodder Education
... maths chapter on page xx gives advice on how to work out the value of maths equations with brackets and combinations of multiplication and ...
... maths chapter on page xx gives advice on how to work out the value of maths equations with brackets and combinations of multiplication and ...
Answers - Pearson-Global
... pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – although in the great majority of cases, there are more than 8 electrons around one atom rather than fewer. Students mig ...
... pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – although in the great majority of cases, there are more than 8 electrons around one atom rather than fewer. Students mig ...
Review Study Guide for the Final
... Define an element. The simplest form of matter that has it a unique set of properties, it cannot be broken down into simpler substances by chemical means ...
... Define an element. The simplest form of matter that has it a unique set of properties, it cannot be broken down into simpler substances by chemical means ...
Mechanistic and Computational Studies of Ferroin, Simple Organic
... Before the year 1950, many chemists in their ‘right mind’ held the archaic belief that all chemical reactions proceed strictly from reactants to products, though some could be coaxed into reverse. What we now know colloquially as a potential energy surface was only visualized in more than two dimens ...
... Before the year 1950, many chemists in their ‘right mind’ held the archaic belief that all chemical reactions proceed strictly from reactants to products, though some could be coaxed into reverse. What we now know colloquially as a potential energy surface was only visualized in more than two dimens ...
Document
... Any property that only depends on object’s current state or condition Independence from method, path or mechanism by which change occurs is important feature of all state functions Some State functions, E, P, t, and V : ...
... Any property that only depends on object’s current state or condition Independence from method, path or mechanism by which change occurs is important feature of all state functions Some State functions, E, P, t, and V : ...
formula writing and nomenclature of inorganic - Parkway C-2
... The two S atoms in the compound must contribute a total charge of +10 in order for the net charge of CaS2O6 to be zero. Therefore, each S atom must have an oxidation number of +5. 3. Determine the oxidation number of Cu in CuSO4. Cu is an element that can have more than one oxidation state (see Tabl ...
... The two S atoms in the compound must contribute a total charge of +10 in order for the net charge of CaS2O6 to be zero. Therefore, each S atom must have an oxidation number of +5. 3. Determine the oxidation number of Cu in CuSO4. Cu is an element that can have more than one oxidation state (see Tabl ...
Chemical bonding and structure
... made of atoms but that there are only about 100 chemically different types of atom. Yet we know that we live in a world made up of literally millions of different substances: somehow these must all be formed from just these 100 atomic building blocks. The extraordinary variety arises from the fact t ...
... made of atoms but that there are only about 100 chemically different types of atom. Yet we know that we live in a world made up of literally millions of different substances: somehow these must all be formed from just these 100 atomic building blocks. The extraordinary variety arises from the fact t ...
Naming Compounds - Kowenscience.com
... the symbol for oxygen, but • take the first part of the element name (the root) and add –ide to get the name oxide. • Since chromium can have more than one charge, a Roman numeral must be used to identify that charge. • There are two oxygen ions each with a 2– charge, giving an overall charge of –4. ...
... the symbol for oxygen, but • take the first part of the element name (the root) and add –ide to get the name oxide. • Since chromium can have more than one charge, a Roman numeral must be used to identify that charge. • There are two oxygen ions each with a 2– charge, giving an overall charge of –4. ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.