Brilliant Preparatory Section, Sitamarhi
... sulphate producing BaSO4 and NaCl is represented by the equation as BaCl2 + Na2SO4 → BaSO4 + NaCl This skeleton equation itself is a balanced one. But in many cases the skeleton equation is not a balanced one. For example, the decomposition of Lead Nitrate giving Lead oxide, NO2 and oxygen. The skel ...
... sulphate producing BaSO4 and NaCl is represented by the equation as BaCl2 + Na2SO4 → BaSO4 + NaCl This skeleton equation itself is a balanced one. But in many cases the skeleton equation is not a balanced one. For example, the decomposition of Lead Nitrate giving Lead oxide, NO2 and oxygen. The skel ...
File
... use the periodic table. All Edexcel’s theory exam papers have a periodic table printed on the back page. The textbook has an identical version inside the back cover. This guide includes a CD-ROM, designed so that answers to individual questions and the explanatory comments can be cut and pasted and ...
... use the periodic table. All Edexcel’s theory exam papers have a periodic table printed on the back page. The textbook has an identical version inside the back cover. This guide includes a CD-ROM, designed so that answers to individual questions and the explanatory comments can be cut and pasted and ...
2E HARRY B. GRAY GEORGE S. HAMMONP.
... not developed in the text for reasons of space and which would normally be taken up in greater detail in later courses. The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Tec ...
... not developed in the text for reasons of space and which would normally be taken up in greater detail in later courses. The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Tec ...
Specification and sample assessment material - Edexcel
... investigative skills based on correct and safe laboratory techniques ...
... investigative skills based on correct and safe laboratory techniques ...
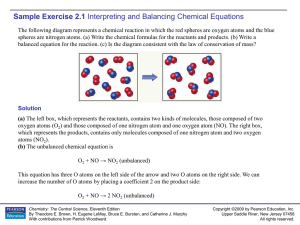
Sample Exercise 2.1
... Because glucose has a formula weight of 180.0 amu, one mole of this substance has a mass of 180.0 g. In other words, C6H12O6 has a molar mass of 180.0 g/mol. Check The magnitude of our answer seems reasonable, and g/mol is the appropriate unit for the molar mass. Comment Glucose is sometimes called ...
... Because glucose has a formula weight of 180.0 amu, one mole of this substance has a mass of 180.0 g. In other words, C6H12O6 has a molar mass of 180.0 g/mol. Check The magnitude of our answer seems reasonable, and g/mol is the appropriate unit for the molar mass. Comment Glucose is sometimes called ...
Chapter - Imperial Valley College
... • compound must have no total charge, therefore we must balance the numbers of cations and anions in a compound to get 0 charge • if Na+ is combined with S2-, you will need 2 Na+ ions for every S2- ion to balance the charges, therefore the formula must be Na2S Tro, Chemistry: A Molecular Approach ...
... • compound must have no total charge, therefore we must balance the numbers of cations and anions in a compound to get 0 charge • if Na+ is combined with S2-, you will need 2 Na+ ions for every S2- ion to balance the charges, therefore the formula must be Na2S Tro, Chemistry: A Molecular Approach ...
Stoichiometry
... • Solid copper(II) oxide reacts with hydrogen gas to form solid copper and liquid water. CuO (s) + H2 (g) ---> Cu (s) + H2O (l) • Aluminum metal reacts with oxygen gas to form solid aluminum oxide. Al (s) + O2 (g) ---> Al2O3 (s) Stoichiometry ...
... • Solid copper(II) oxide reacts with hydrogen gas to form solid copper and liquid water. CuO (s) + H2 (g) ---> Cu (s) + H2O (l) • Aluminum metal reacts with oxygen gas to form solid aluminum oxide. Al (s) + O2 (g) ---> Al2O3 (s) Stoichiometry ...
Ions
... Chapter 6 Why do atoms react? • It is this arrangement of electrons that imparts stability to the noble gases • All other elements react in order to achieve the same electron configuration as their nearest noble gas neighbor (8 valence e- = octet rule) • Atoms can gain, lose, or share electrons in ...
... Chapter 6 Why do atoms react? • It is this arrangement of electrons that imparts stability to the noble gases • All other elements react in order to achieve the same electron configuration as their nearest noble gas neighbor (8 valence e- = octet rule) • Atoms can gain, lose, or share electrons in ...
The Precautionary Principle and chemical risks - Hal-SHS
... With this background, scientific uncertainty may affect different points of risky situations. We may have uncertainty in the realization of harm: harm is then just a possibility which has been neither dismissed nor proven. We may also have uncertainty in the causes of a given phenomenon: damage to h ...
... With this background, scientific uncertainty may affect different points of risky situations. We may have uncertainty in the realization of harm: harm is then just a possibility which has been neither dismissed nor proven. We may also have uncertainty in the causes of a given phenomenon: damage to h ...
Chapter 09 An Overview of Chemical Reactions Notes
... CaCO3 (s) + 2 HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 (g) a. How many moles of HCl (aq) are required to react with 2.5 mol of calcium carbonate? b. How many moles of carbon dioxide would be produced if 2.5 mol of calcium carbonate is used? 4. Aluminum reacts with hydrochloric acid, HCl (aq), to produc ...
... CaCO3 (s) + 2 HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 (g) a. How many moles of HCl (aq) are required to react with 2.5 mol of calcium carbonate? b. How many moles of carbon dioxide would be produced if 2.5 mol of calcium carbonate is used? 4. Aluminum reacts with hydrochloric acid, HCl (aq), to produc ...
Chapter 23 + Practice Problems - Bloomsburg Area School District
... have a large amount of saturated fatty acids, fats are solids at room temperature. Oils have more unsaturated fatty acids than fats, and are liquids. Like other animals, humans make fat, which is stored in adipose tissue until it is needed as an energy source. Fat has about twice as much energy per ...
... have a large amount of saturated fatty acids, fats are solids at room temperature. Oils have more unsaturated fatty acids than fats, and are liquids. Like other animals, humans make fat, which is stored in adipose tissue until it is needed as an energy source. Fat has about twice as much energy per ...
CHEM 121 Chp 5 Spaulding
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
Organizing Topic - Staunton City Schools
... The Science Standards of Learning Enhanced Scope and Sequence is a resource intended to help teachers align their classroom instruction with the Science Standards of Learning that were adopted by the Board of Education in January 2003. The Enhanced Scope and Sequence contains the following: Units or ...
... The Science Standards of Learning Enhanced Scope and Sequence is a resource intended to help teachers align their classroom instruction with the Science Standards of Learning that were adopted by the Board of Education in January 2003. The Enhanced Scope and Sequence contains the following: Units or ...
Procedure - Loudoun County Public Schools
... The mixture for the “Percent Sand, Salt, Iron Filings, Mystery Substance in a Mixture” lab activity will need to be prepared prior to the beginning of the activity. Because the purpose of the activity is to practice safe techniques in the laboratory, exact measurements for the mixture are not necess ...
... The mixture for the “Percent Sand, Salt, Iron Filings, Mystery Substance in a Mixture” lab activity will need to be prepared prior to the beginning of the activity. Because the purpose of the activity is to practice safe techniques in the laboratory, exact measurements for the mixture are not necess ...
Textbook sample chapter
... covalently bonded. Many of the formulae that you meet in this course have giant structures with ionic or covalent bonding. Sodium chloride has ionic bonding and consists of a large number of sodium ions and an equally large number of chloride ions held together in a lattice by electrostatic charges. ...
... covalently bonded. Many of the formulae that you meet in this course have giant structures with ionic or covalent bonding. Sodium chloride has ionic bonding and consists of a large number of sodium ions and an equally large number of chloride ions held together in a lattice by electrostatic charges. ...
mole - hrsbstaff.ednet.ns.ca
... that represents a number, a dozen, which represent 12. The role of the mole in chemistry is that of counting ions, atoms or molecules. The chemist can "count" ions, atoms or molecules by weighing very large numbers of them to get a significant mass. One mole contains as many atoms as there are in 12 ...
... that represents a number, a dozen, which represent 12. The role of the mole in chemistry is that of counting ions, atoms or molecules. The chemist can "count" ions, atoms or molecules by weighing very large numbers of them to get a significant mass. One mole contains as many atoms as there are in 12 ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.