Experimental skills and abilities
... 2 Answers could include references to the different temperatures and pressures at which the experiment was carried out compared to the standard conditions of 25 oC (298 K) and 1 atmosphere of pressure. ...
... 2 Answers could include references to the different temperatures and pressures at which the experiment was carried out compared to the standard conditions of 25 oC (298 K) and 1 atmosphere of pressure. ...
Notes for Quarter I
... Visible Light: These EM waves cover a very narrow range on the spectrum that humans can see. They have wavelengths between 400 nm and 700 nm. Some of the sun’s energy that reaches Earth is visible light. This visible light is white light, and contains all wavelengths of visible light combined. We s ...
... Visible Light: These EM waves cover a very narrow range on the spectrum that humans can see. They have wavelengths between 400 nm and 700 nm. Some of the sun’s energy that reaches Earth is visible light. This visible light is white light, and contains all wavelengths of visible light combined. We s ...
Towards a Theory of Organizations
... 2. Algebra of Organizations In this contribution we will only concentrate on the static structure of organizations and will neglect their dynamics. For this reason we introduce the concept of an algebraic chemistry. An algebraic chemistry is a specific artificial chemistry without any dynamics. Defi ...
... 2. Algebra of Organizations In this contribution we will only concentrate on the static structure of organizations and will neglect their dynamics. For this reason we introduce the concept of an algebraic chemistry. An algebraic chemistry is a specific artificial chemistry without any dynamics. Defi ...
M - coercingmolecules
... Cu2S) by a multistep process. After an initial grinding, the first step is to “roast” the ore (heat it strongly with O2) to form Cu2O and SO2 2Cu2S(s) + 3O2(g) ...
... Cu2S) by a multistep process. After an initial grinding, the first step is to “roast” the ore (heat it strongly with O2) to form Cu2O and SO2 2Cu2S(s) + 3O2(g) ...
Chapter 3
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
Ionic Compounds
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
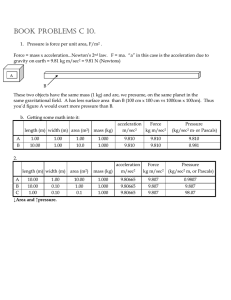
book problems c 10.
... of six children. Avogadro led an industrious life, and was a modest man, working in isolation. This probably contributed to his relative obscurity, particularly outside Italy. Avogadro died on the 9th July, 1856. He was described as religious, but not a bigot. Avogadro - his contribution to chemistr ...
... of six children. Avogadro led an industrious life, and was a modest man, working in isolation. This probably contributed to his relative obscurity, particularly outside Italy. Avogadro died on the 9th July, 1856. He was described as religious, but not a bigot. Avogadro - his contribution to chemistr ...
compounds - Belle Vernon Area
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
... • Ionic bonds—which occur between metals and nonmetals—involve the transfer of electrons from one atom to another. • When a metal interacts with a nonmetal, it can transfer one or more of its electrons to the nonmetal. – The metal atom then becomes a cation. – The nonmetal atom becomes an anion. ...
Worksheet Significant Figures
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
the chemistry of life: organic and biological chemistry
... understand the chemical behaviors of molecules of low molar mass. This chapter is about the molecules, composed mainly of carbon, hydrogen, oxygen, and nitrogen, that form the basis of both organic and biological chemistry. The element carbon forms a vast number of compounds. Over 16 million carbon- ...
... understand the chemical behaviors of molecules of low molar mass. This chapter is about the molecules, composed mainly of carbon, hydrogen, oxygen, and nitrogen, that form the basis of both organic and biological chemistry. The element carbon forms a vast number of compounds. Over 16 million carbon- ...
Harrisburg Area Community College 2013/2014
... Data: ........................................................................................................................................................17 Separation of Mixtures .................................................................................................................... ...
... Data: ........................................................................................................................................................17 Separation of Mixtures .................................................................................................................... ...
2 Atoms and Molecules
... In Chapter 1, we defined elements as homogeneous pure substances made up of identical atoms. At least 115 different elements are known to exist. This leads to the conclusion that a minimum of 115 different kinds of atoms exist. Eighty-eight of the elements are naturally occurring and therefore are f ...
... In Chapter 1, we defined elements as homogeneous pure substances made up of identical atoms. At least 115 different elements are known to exist. This leads to the conclusion that a minimum of 115 different kinds of atoms exist. Eighty-eight of the elements are naturally occurring and therefore are f ...
Introduction to Inorganic Chemistry
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...
LECTURE 5 - CHEMICAL EQUILIBRIUM
... must be altered so that K remains constant. Because [B] has increased, the reaction must proceed to the right, thus reducing both [A] and [B], and increasing [C] and [D]. When the right side is equal to the original value, the system is again at equilibrium. If more C had been added to the original ...
... must be altered so that K remains constant. Because [B] has increased, the reaction must proceed to the right, thus reducing both [A] and [B], and increasing [C] and [D]. When the right side is equal to the original value, the system is again at equilibrium. If more C had been added to the original ...
Chemistry Transition Information
... that substance). You cannot change the formulas (this would make a different substance). Hint – start with unbalanced elements that only appear in one substance on each side of the equation. Keep doing this until the equation is balanced. ...
... that substance). You cannot change the formulas (this would make a different substance). Hint – start with unbalanced elements that only appear in one substance on each side of the equation. Keep doing this until the equation is balanced. ...
8 theoretical problems 2 practical problems
... excess acidic phenylhydrazine. Compound C reacts with nitric acid to give an optically inactive compound D. The Kiliani-Fischer approach is used to establish the configurational relationship between D-glyceraldehyde and C. The intermediate aldotetrose which leads to C does not give a meso compound w ...
... excess acidic phenylhydrazine. Compound C reacts with nitric acid to give an optically inactive compound D. The Kiliani-Fischer approach is used to establish the configurational relationship between D-glyceraldehyde and C. The intermediate aldotetrose which leads to C does not give a meso compound w ...
Ch 3 Student.pptx
... 1. The cation is always named first and the anion second. 2. Cation takes its name from the name of the parent element. 3. Anion is named by taking the root of the element name and adding –ide. ...
... 1. The cation is always named first and the anion second. 2. Cation takes its name from the name of the parent element. 3. Anion is named by taking the root of the element name and adding –ide. ...
WIPO IPC: Internet Publication
... and a non-chemical part or aspect, the general rule is that the chemical part or aspect is covered by section C. In some of these cases, the chemical part or aspect brings with it a non-chemical one, even though purely mechanical, because this latter aspect either is essential to the operation or tr ...
... and a non-chemical part or aspect, the general rule is that the chemical part or aspect is covered by section C. In some of these cases, the chemical part or aspect brings with it a non-chemical one, even though purely mechanical, because this latter aspect either is essential to the operation or tr ...
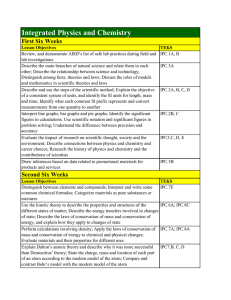
Integrated Physics and Chemistry
... Distinguish between elements and compounds; Interpret and write some common chemical formulas; Categorize materials as pure substances or mixtures Use the kinetic theory to describe the properties and structures of the different states of matter; Describe the energy transfers involved in changes of ...
... Distinguish between elements and compounds; Interpret and write some common chemical formulas; Categorize materials as pure substances or mixtures Use the kinetic theory to describe the properties and structures of the different states of matter; Describe the energy transfers involved in changes of ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.