Chapter
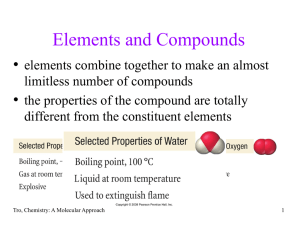
... • compound must have no total charge, therefore we must balance the numbers of cations and anions in a compound to get 0 charge • if Na+ is combined with S2-, you will need 2 Na+ ions for every S2- ion to balance the charges, therefore the formula must be Na2S Tro, Chemistry: A Molecular Approach ...
... • compound must have no total charge, therefore we must balance the numbers of cations and anions in a compound to get 0 charge • if Na+ is combined with S2-, you will need 2 Na+ ions for every S2- ion to balance the charges, therefore the formula must be Na2S Tro, Chemistry: A Molecular Approach ...
Unit-2-Hydrocarbons
... • Esters, on the other hand, produce the sweet, often pleasant order associated with flowers, perfumes and various natural and artificial flavorings. The next slide shows Figure 4.24 from Raymond, which gives some specific examples. ...
... • Esters, on the other hand, produce the sweet, often pleasant order associated with flowers, perfumes and various natural and artificial flavorings. The next slide shows Figure 4.24 from Raymond, which gives some specific examples. ...
Chemistry - Separation techniques
... Further details are available in the specifications (Practical Skills Topics), and in these videos. ...
... Further details are available in the specifications (Practical Skills Topics), and in these videos. ...
AP Chemistry - Oak Park Unified School District
... are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic units of length, mass, and time, respectively. The SI temperature scale is the (5) scale, although t ...
... are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic units of length, mass, and time, respectively. The SI temperature scale is the (5) scale, although t ...
Chapter22_LEC
... • large atom and weaker oxidizer than oxygen • often shows +2, +4, or +6 oxidation numbers in its ...
... • large atom and weaker oxidizer than oxygen • often shows +2, +4, or +6 oxidation numbers in its ...
Chapter - WTPS.org
... • large atom and weaker oxidizer than oxygen • often shows +2, +4, or +6 oxidation numbers in its ...
... • large atom and weaker oxidizer than oxygen • often shows +2, +4, or +6 oxidation numbers in its ...
Molecular Compound
... cannot fit eight electrons, and for those that can fit more than eight electrons, into their outermost orbital • Hydrogen forms bonds in which it is surrounded by only two electrons • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons • Main- ...
... cannot fit eight electrons, and for those that can fit more than eight electrons, into their outermost orbital • Hydrogen forms bonds in which it is surrounded by only two electrons • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons • Main- ...
Chemical Equations and Reactions
... must be identified, either through chemical analysis in the laboratory or from sources that give the results of experiments. 2. The equation must contain the correct formulas for the reactants and products. Remember what you learned in Chapter 7 about symbols and formulas. Knowledge of the common ox ...
... must be identified, either through chemical analysis in the laboratory or from sources that give the results of experiments. 2. The equation must contain the correct formulas for the reactants and products. Remember what you learned in Chapter 7 about symbols and formulas. Knowledge of the common ox ...
Term 111, Final Exam (All correct choices are A): 1. What is the
... B) at some angle larger than 0 and less than 90 degrees C) sharing the same space D) at some angle larger than 120 and less than 180 degrees E) coplanar (at a 0 degree angle) to each other Choice A ...
... B) at some angle larger than 0 and less than 90 degrees C) sharing the same space D) at some angle larger than 120 and less than 180 degrees E) coplanar (at a 0 degree angle) to each other Choice A ...
Syllabus Advanced Level and Advanced Subsidiary Level
... Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
... Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
Chapter Two - Blackboard
... the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only water to the bucket, and so he concluded that all th ...
... the mass of a young willow tree and, separately, the mass of a bucket of soil and then planted the tree in the bucket. After five years, he found that the tree had gained 75 kg in mass even though the soil had lost only 0.057 kg. He had added only water to the bucket, and so he concluded that all th ...
base hydrolysis of cobalt(iii)
... However, a mixture of [Coen 2 NO 2 Cl], NO 2 - , and OH readily afford [Coen 2 (NO 2 ) 2 ]+ which must occur without the intermediate formation of [Coen 2 NO 2 OH]+ required, were this to involve an S N 2 pathway. The role of the OH - here is in keeping with an S N 1CB mechanism. Although these expe ...
... However, a mixture of [Coen 2 NO 2 Cl], NO 2 - , and OH readily afford [Coen 2 (NO 2 ) 2 ]+ which must occur without the intermediate formation of [Coen 2 NO 2 OH]+ required, were this to involve an S N 2 pathway. The role of the OH - here is in keeping with an S N 1CB mechanism. Although these expe ...
CHEMISTRY CHM-050 Introduction to Chemistry I NCC Cr: 3 D Lec
... CHM-122 Introduction to General Chemistry NIACC Cr: 4 A Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, e ...
... CHM-122 Introduction to General Chemistry NIACC Cr: 4 A Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, e ...
ض ( ا ء ا ط ك ا رر 108 1) -
... Which state of the matter has definite volume and indefinite shape? solid B plasma C liquid D gas Which state of the matter has indefinite volume and indefinite shape? ...
... Which state of the matter has definite volume and indefinite shape? solid B plasma C liquid D gas Which state of the matter has indefinite volume and indefinite shape? ...
Some basic concepts of chemistry
... Chemistry is one of the oldest academic discipline and its roots lie in man’s fascination towards study of structure, composition and properties of matter and the reactions by which matter converts from one form to the other. NEET: Chemistry (Vol. I) not only adds great value towards a progressive s ...
... Chemistry is one of the oldest academic discipline and its roots lie in man’s fascination towards study of structure, composition and properties of matter and the reactions by which matter converts from one form to the other. NEET: Chemistry (Vol. I) not only adds great value towards a progressive s ...
unit (4) calculations and chemical reactions
... H2O = (2 x 1.01 amu) + (1 x 16.00 amu) = 18.02 amu N2O3 = (2 x 14.01 amu) + (3 x 16.00 amu) = 76.02 amu Mg(OH)2 = (1 x 24.31 amu) + (2 x 1.01 amu) + (2 x 16.00 amu) = 58.33 amu The formula mass of H2O is 18.02 amu so the molar mass is 18.02 g/mol. The formula mass of N2O3 is 76.02 amu so the molar m ...
... H2O = (2 x 1.01 amu) + (1 x 16.00 amu) = 18.02 amu N2O3 = (2 x 14.01 amu) + (3 x 16.00 amu) = 76.02 amu Mg(OH)2 = (1 x 24.31 amu) + (2 x 1.01 amu) + (2 x 16.00 amu) = 58.33 amu The formula mass of H2O is 18.02 amu so the molar mass is 18.02 g/mol. The formula mass of N2O3 is 76.02 amu so the molar m ...
Midterm Practice Exam Key
... 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which the aqueous (dissolved) ions ...
... 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which the aqueous (dissolved) ions ...
127 - Chimica
... Summary: The reaction of [Re,(CO),,] with OH- gives first [Re,H(CO),]- and then, under more drastic conditions, the novel anion [Re2H,(CO),I2-, which can be reversibly protonated to give [ Re,H,(p-H)(CO),]-, whose structure has been elucidated by a X-ray investigation. Variable-temperature NMR spect ...
... Summary: The reaction of [Re,(CO),,] with OH- gives first [Re,H(CO),]- and then, under more drastic conditions, the novel anion [Re2H,(CO),I2-, which can be reversibly protonated to give [ Re,H,(p-H)(CO),]-, whose structure has been elucidated by a X-ray investigation. Variable-temperature NMR spect ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble NaCl is soluble in water, AgCl is insoluble in water the degree of solubility depends on the temperature even insoluble compounds dissolve, just not enough to be ...
... compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble NaCl is soluble in water, AgCl is insoluble in water the degree of solubility depends on the temperature even insoluble compounds dissolve, just not enough to be ...
ROCZNIKI FILOZOFICZNE Tom XXXI, zeszyt 3 — 1983
... compounds, may be considered. The hypothesis on the role of Si-etioporphyrins as initial compounds in early phases of the evolution of protoliving systems, was put forward by Sedlak (23) in his approach to paleobiochemistry of silicon. This problem seems to be of importance, because the possible rol ...
... compounds, may be considered. The hypothesis on the role of Si-etioporphyrins as initial compounds in early phases of the evolution of protoliving systems, was put forward by Sedlak (23) in his approach to paleobiochemistry of silicon. This problem seems to be of importance, because the possible rol ...
Pirogov National Medical Univercity of Vinnitsa
... places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his foreign objects that are unrelated to this study. Disorder and carelessness in carryi ...
... places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his foreign objects that are unrelated to this study. Disorder and carelessness in carryi ...
Chapter 7
... Hess’s law of constant heat summation If a process occurs in stages or steps (even hypothetically), the enthalpy change for the overall process is the sum of the enthalpy changes for the individual steps. ½N2(g) + ½O2(g) → NO(g) ...
... Hess’s law of constant heat summation If a process occurs in stages or steps (even hypothetically), the enthalpy change for the overall process is the sum of the enthalpy changes for the individual steps. ½N2(g) + ½O2(g) → NO(g) ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.