LEP 5.1.03 -15 Franck-Hertz experiment with Ne-tube
... 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Plan ...
... 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Plan ...
Hwk Set #14 - Publisher`s solutions
... Assess: This answer is one-fourth of the answer in Example 41.9, which makes sense. An electron would emit a 1200 nm photon in any n → n − 1 jump in this quantum harmonic oscillator; not just the 3 → 2 jump. ...
... Assess: This answer is one-fourth of the answer in Example 41.9, which makes sense. An electron would emit a 1200 nm photon in any n → n − 1 jump in this quantum harmonic oscillator; not just the 3 → 2 jump. ...
Regents questions
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
Document
... (b) magnitude of the momentum, and (c) wavelength of the photon emitted when a hydrogen atom undergoes a transition from a state with n = 3 to a state with n = 1? ANSWER: (a) 12.1 eV; (b) 6.45 10-27 kg.m/s; (c) 102 nm 4. How much work must be done to pull apart the electron and the proton that mak ...
... (b) magnitude of the momentum, and (c) wavelength of the photon emitted when a hydrogen atom undergoes a transition from a state with n = 3 to a state with n = 1? ANSWER: (a) 12.1 eV; (b) 6.45 10-27 kg.m/s; (c) 102 nm 4. How much work must be done to pull apart the electron and the proton that mak ...
2. NH3 - Huffman Chemistry Website!
... Complete the following table. Write the chemical name and the chemical formula for the compounds formed. ...
... Complete the following table. Write the chemical name and the chemical formula for the compounds formed. ...
PowerPoint Presentation - Duality of Matter
... The wave nature makes it impossible to know with infinite precision how atomic matter moves. Specifically: To know a particle’s motion we must know its position and velocity at the same time. But how do you locate the position of a wave/particle electron? ...
... The wave nature makes it impossible to know with infinite precision how atomic matter moves. Specifically: To know a particle’s motion we must know its position and velocity at the same time. But how do you locate the position of a wave/particle electron? ...
Light and quantized Energy Section 1
... The excited state n=2 to its Ground state n=1 by emitting (releasing) energy as a photon ...
... The excited state n=2 to its Ground state n=1 by emitting (releasing) energy as a photon ...
科目名 Course Title Extreme Laser Physics [極限レーザー物理E] 講義
... Interactions between optical field and atomic, molecular, and materials system have been providing interesting issues in physics. This course covers the basics of ultrafast optics and atomic physics, necessary to understand rapidly growing research field in atomic, molecular, and optical physics, wi ...
... Interactions between optical field and atomic, molecular, and materials system have been providing interesting issues in physics. This course covers the basics of ultrafast optics and atomic physics, necessary to understand rapidly growing research field in atomic, molecular, and optical physics, wi ...
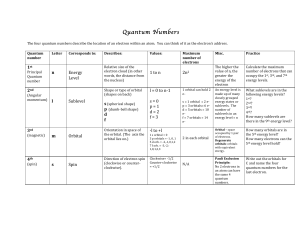
Quantum Numbers Handout File
... occupied!by!1!pair! of!electrons.! Degenerate' orbitals:!orbitals! with!equivalent! energy.!! ...
... occupied!by!1!pair! of!electrons.! Degenerate' orbitals:!orbitals! with!equivalent! energy.!! ...
64-311/5: Atomic and Molecular Spectra
... Into how many different energy levels will the n = 3 split? Label the different levels and determine their relative spacing in electron-volts. 10 (a) By combining spin and orbital angular momentum, determine the different possible J values for a d electron within the LS coupling scheme. What are the ...
... Into how many different energy levels will the n = 3 split? Label the different levels and determine their relative spacing in electron-volts. 10 (a) By combining spin and orbital angular momentum, determine the different possible J values for a d electron within the LS coupling scheme. What are the ...
The Modern Atomic Model
... • sublevels contain the actual atomic orbitals (locations for e -) • s-sublevel– 1 orbital, shaped like a sphere • p-sublevel– 3 orbitals, shaped like a dumbbell • d-sublevel– 5 orbitals, irregular shape • f-sublevel– 7 orbitals, irregular shape ...
... • sublevels contain the actual atomic orbitals (locations for e -) • s-sublevel– 1 orbital, shaped like a sphere • p-sublevel– 3 orbitals, shaped like a dumbbell • d-sublevel– 5 orbitals, irregular shape • f-sublevel– 7 orbitals, irregular shape ...
Orbitals and energy levels
... The timeline shoes the development of atomic models from 1803 to 1911. ...
... The timeline shoes the development of atomic models from 1803 to 1911. ...
Slide 1
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
Chemistry 102 Summary June 25th - Bohr model only works for one
... Specific wave functions are called orbitals. Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be d ...
... Specific wave functions are called orbitals. Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be d ...












![科目名 Course Title Extreme Laser Physics [極限レーザー物理E] 講義](http://s1.studyres.com/store/data/003538965_1-4c9ae3641327c1116053c260a01760fe-300x300.png)