HNRS 227 Lecture #2 Chapters 2 and 3
... When people refer to the “Calorie content of food,” they are referring to the amount of chemical energy available from the food. One way to measure the chemical energy of foodstuff is to find out how much heat is released by complete oxidation. A Calorie (kcal) is a measure of the heat release and ...
... When people refer to the “Calorie content of food,” they are referring to the amount of chemical energy available from the food. One way to measure the chemical energy of foodstuff is to find out how much heat is released by complete oxidation. A Calorie (kcal) is a measure of the heat release and ...
First Law of Thermodynamics - Erwin Sitompul
... Instead, the sample may change from one phase, or state, to another, with no change in temperature. The amount of energy per unit mass that must be transferred as heat when a sample completely undergoes a phase change is called the heat of transformation L. Thus, when a sample of mass m comple ...
... Instead, the sample may change from one phase, or state, to another, with no change in temperature. The amount of energy per unit mass that must be transferred as heat when a sample completely undergoes a phase change is called the heat of transformation L. Thus, when a sample of mass m comple ...
HNRS 227 Lecture #2 Chapters 2 and 3
... When people refer to the “Calorie content of food,” they are referring to the amount of chemical energy available from the food. One way to measure the chemical energy of foodstuff is to find out how much heat is released by complete oxidation. A Calorie (kcal) is a measure of the heat release and t ...
... When people refer to the “Calorie content of food,” they are referring to the amount of chemical energy available from the food. One way to measure the chemical energy of foodstuff is to find out how much heat is released by complete oxidation. A Calorie (kcal) is a measure of the heat release and t ...
H = 890kJ - George Mason University
... Work is defined as force acting over some distance: w = d x F (referenced to the system). During reactions often there is an expansion of gases against some pressure where pressure is equal to the force per unit area: F P or F PxA . A Work is obtained by substitution: w = d x F = d x ( ...
... Work is defined as force acting over some distance: w = d x F (referenced to the system). During reactions often there is an expansion of gases against some pressure where pressure is equal to the force per unit area: F P or F PxA . A Work is obtained by substitution: w = d x F = d x ( ...
Unit 61: Engineering Thermodynamics
... • The quantity U + PV is known as enthalpy (H). As this is a combination of properties, it itself is therefore a property. • Specific enthalpy is found by dividing by the mass… h = u + Pv • Thus Q1-2 = H2 – H1 • Note: the enthalpy was defined using a constantpressure system with the differences betw ...
... • The quantity U + PV is known as enthalpy (H). As this is a combination of properties, it itself is therefore a property. • Specific enthalpy is found by dividing by the mass… h = u + Pv • Thus Q1-2 = H2 – H1 • Note: the enthalpy was defined using a constantpressure system with the differences betw ...
Thermodynamics
... equilibrium with C and B in thermal equilibrium with C then A and B have to be in thermal equilibrium. No heat flows! ...
... equilibrium with C and B in thermal equilibrium with C then A and B have to be in thermal equilibrium. No heat flows! ...
IOSR Journal of Mathematics (IOSR-JM)
... release heat for convection more slowly than the transparent medium. It is also shown that radiation affects the cell size at the onset of convection only in the case of transparent medium. Anwar et al. [12] studied the effects of thermal radiation and porous drag forces on the natural convection he ...
... release heat for convection more slowly than the transparent medium. It is also shown that radiation affects the cell size at the onset of convection only in the case of transparent medium. Anwar et al. [12] studied the effects of thermal radiation and porous drag forces on the natural convection he ...
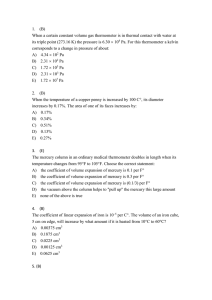
Quiz_MATH.rtf
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
ENGINEERING_THERMODYNAMICS
... It is the transfer of heat within a fluid or gas or between two mediums by the motion of molecules from one region to another region. Water heated up in a vessel is an example for convection heat transfer. 3) Radiation : It is the transfer of heat from hot body to cold body by movement particles in ...
... It is the transfer of heat within a fluid or gas or between two mediums by the motion of molecules from one region to another region. Water heated up in a vessel is an example for convection heat transfer. 3) Radiation : It is the transfer of heat from hot body to cold body by movement particles in ...
AP Physics - Thermodynamics
... Heading up the do-not camp was Stuart Nelson Jr., head veterinarian for the famous Iditarod dogsled race currently under way in Alaska. This 1,100-mile event lasts two weeks and features several dozen dog teams and their mushers racing from Anchorage to Nome in some of the most grueling conditions i ...
... Heading up the do-not camp was Stuart Nelson Jr., head veterinarian for the famous Iditarod dogsled race currently under way in Alaska. This 1,100-mile event lasts two weeks and features several dozen dog teams and their mushers racing from Anchorage to Nome in some of the most grueling conditions i ...
revision - metc instructors collab site
... Explains that R will have a different numerical value for each ideal gas or mixture of Ideal gases ...
... Explains that R will have a different numerical value for each ideal gas or mixture of Ideal gases ...
heat engine
... Conceptual Example 8 Natural Limits on the Efficiency of a Heat Engine Consider a hypothetical engine that receives 1000 J of heat as input from a hot reservoir and delivers 1000J of work, rejecting no heat to a cold reservoir whose temperature is above 0 K. Decide whether this engine violates the f ...
... Conceptual Example 8 Natural Limits on the Efficiency of a Heat Engine Consider a hypothetical engine that receives 1000 J of heat as input from a hot reservoir and delivers 1000J of work, rejecting no heat to a cold reservoir whose temperature is above 0 K. Decide whether this engine violates the f ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.