Thermodynamics - WordPress.com
... Energy (Joule) • Energy can be transferred or transformed • kinetic • potential (chemical, electrical, gravitational, elastic) • radiant (sound, light and other electromagnetic waves) • internal (heat/thermal energy) ...
... Energy (Joule) • Energy can be transferred or transformed • kinetic • potential (chemical, electrical, gravitational, elastic) • radiant (sound, light and other electromagnetic waves) • internal (heat/thermal energy) ...
Thermodynamics
... In every case, we assume that the process occurs “slowly.” The technical term for “occurs slowly” is quasi-static. What does “slowly” mean? It means that the system has time to mix during the process. At all times, we consider the temperature and the pressure of the system to be uniform (the same in ...
... In every case, we assume that the process occurs “slowly.” The technical term for “occurs slowly” is quasi-static. What does “slowly” mean? It means that the system has time to mix during the process. At all times, we consider the temperature and the pressure of the system to be uniform (the same in ...
Chapter 4
... • Temperature is a measure of heat content of atoms and molecules. • The higher the temperature the more heat the atoms and/or molecules of the substance contain. • The higher the heat and temperature the higher the average kinetic energy of the atoms and molecules of the substance (element or compo ...
... • Temperature is a measure of heat content of atoms and molecules. • The higher the temperature the more heat the atoms and/or molecules of the substance contain. • The higher the heat and temperature the higher the average kinetic energy of the atoms and molecules of the substance (element or compo ...
Final exam questions for Chemical Engineer BSc
... ideal gas law. Non-ideal gases: the compression factor, the virial equation, the van der Waals equation and its parameters, critical point. Fundamentals of the kinetic theory of gases. The molecular origin of pressure. The Maxwell distribution of speeds for gases. 2. The zeroth and the first law of ...
... ideal gas law. Non-ideal gases: the compression factor, the virial equation, the van der Waals equation and its parameters, critical point. Fundamentals of the kinetic theory of gases. The molecular origin of pressure. The Maxwell distribution of speeds for gases. 2. The zeroth and the first law of ...
Kémiai technológia I
... ideal gas law. Non-ideal gases: the compression factor, the virial equation, the van der Waals equation and its parameters, critical point. Fundamentals of the kinetic theory of gases. The molecular origin of pressure. The Maxwell distribution of speeds for gases. 2. The zeroth and the first law of ...
... ideal gas law. Non-ideal gases: the compression factor, the virial equation, the van der Waals equation and its parameters, critical point. Fundamentals of the kinetic theory of gases. The molecular origin of pressure. The Maxwell distribution of speeds for gases. 2. The zeroth and the first law of ...
Physical Chemistry for the Biosciences I (Ch 416 )
... gravitational, mechanical etc. Displacement does not have to be a physical distance; it can be an angle for example, But in thermodynamic context, we have to define work bit more precisely. Note the force is the external force, meaning force exerted by the surrounding on the system. If there is no e ...
... gravitational, mechanical etc. Displacement does not have to be a physical distance; it can be an angle for example, But in thermodynamic context, we have to define work bit more precisely. Note the force is the external force, meaning force exerted by the surrounding on the system. If there is no e ...
THE IMP ACT OF METEORS` By John D. Boon Energy changes that
... Desert. The sand was already largely pulverized, and incapable of transmitting waves, hence great heat became necessary in the dissipation of the energy. In all elastic bodies whether brittle or not, a considerable portion of the energy of an impact is dissipated by means of waves that run through t ...
... Desert. The sand was already largely pulverized, and incapable of transmitting waves, hence great heat became necessary in the dissipation of the energy. In all elastic bodies whether brittle or not, a considerable portion of the energy of an impact is dissipated by means of waves that run through t ...
ENGR-440, Chemical Engineering Thermodynamics 1 2001
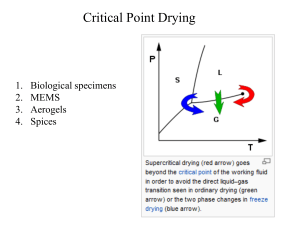
... 2. To be able to relate the pVT properties of a pure substance. 1. Define pressure, temperature and specific volume. 2. Draw pressure-temperature, pressure-specific volume, and temperature-specific volume phase diagrams. 3. Use the Ideal Gas Law, the Virial Coefficient Equation of State, the Redlick ...
... 2. To be able to relate the pVT properties of a pure substance. 1. Define pressure, temperature and specific volume. 2. Draw pressure-temperature, pressure-specific volume, and temperature-specific volume phase diagrams. 3. Use the Ideal Gas Law, the Virial Coefficient Equation of State, the Redlick ...
Second Law of thermodynamics
... • It is one of the great laws of physics • Its validity rests on experiments (such as Joule’s) in which no exceptions have been seen • Internal energy is the sum total of all the energy the molecules of the system. It is a property of a system like pressure, volume and temperature • Work and heat ar ...
... • It is one of the great laws of physics • Its validity rests on experiments (such as Joule’s) in which no exceptions have been seen • Internal energy is the sum total of all the energy the molecules of the system. It is a property of a system like pressure, volume and temperature • Work and heat ar ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.