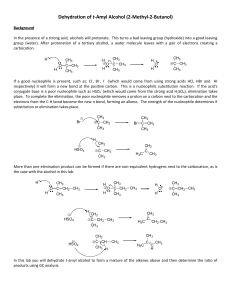
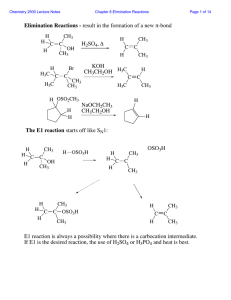
DEPARTMENT OF CHEMISTRY, UNIVERSITY OF JYVÄSKYLÄ
... compounds. Hence, understanding the rules governing the formation of chemical bonds has been a priority for chemists for hundreds of years. The modern description of chemical bonding is based on quantum theory and the atomic shell structure, which easily rationalize why bonding is typically far simp ...
... compounds. Hence, understanding the rules governing the formation of chemical bonds has been a priority for chemists for hundreds of years. The modern description of chemical bonding is based on quantum theory and the atomic shell structure, which easily rationalize why bonding is typically far simp ...
lecture 7 reductive eliminations
... as a σ complex and then undergoes bond breaking as a result of strong back donation from the metal into the * orbital. • Non‐polar reagents, such as H2, or compounds containing C−H and Si−H bonds all tend to react via a σ complex transition state (or even an intermediate) of this type. • The associ ...
... as a σ complex and then undergoes bond breaking as a result of strong back donation from the metal into the * orbital. • Non‐polar reagents, such as H2, or compounds containing C−H and Si−H bonds all tend to react via a σ complex transition state (or even an intermediate) of this type. • The associ ...
amine cured-epoxy matrices
... Epoxy resins can be cured with a variety of compounds called curing agents which are also known as curatives, hardeners, or converters. Of the many classes/types of curing agents, amines are most widely utilized as curing agents in epoxy matrices for high performance composites. This produces a hete ...
... Epoxy resins can be cured with a variety of compounds called curing agents which are also known as curatives, hardeners, or converters. Of the many classes/types of curing agents, amines are most widely utilized as curing agents in epoxy matrices for high performance composites. This produces a hete ...
BSA + TMCS + TMSI - Sigma
... Silylation is the most widely used derivatization procedure for GC analysis. In silylation, an active hydrogen is replaced by an alkylsilyl group, most often trimethylsilyl (TMS). Compared to their parent compounds, silyl derivatives generally are more volatile, less polar, and more thermally stable ...
... Silylation is the most widely used derivatization procedure for GC analysis. In silylation, an active hydrogen is replaced by an alkylsilyl group, most often trimethylsilyl (TMS). Compared to their parent compounds, silyl derivatives generally are more volatile, less polar, and more thermally stable ...
1.7AMINES
... amines is weaker than in alcohols of related formulas. As a result, the boiling points of amines are lower than those of the related alcohols. 3. Amines are weak bases. Amines have a relatively high solubility in water because of the hydrogen bonding that occurs in primary and secondary amines. Tert ...
... amines is weaker than in alcohols of related formulas. As a result, the boiling points of amines are lower than those of the related alcohols. 3. Amines are weak bases. Amines have a relatively high solubility in water because of the hydrogen bonding that occurs in primary and secondary amines. Tert ...
Richard R. Schrock - Nobel Lecture
... yield a metal-hydride and an alkene. The relative stabilities of high oxidation state “homoleptic” or “peralkyl” compounds such as M[CH2Si(CH3)3]4, M(CH2C6H5)4, and M[CH2C(CH3)3]4 (M = Ti, Zr, or Hf; Fig 2), were rationalized on the basis of the fact that unlike a compound having an ethyl ligand, th ...
... yield a metal-hydride and an alkene. The relative stabilities of high oxidation state “homoleptic” or “peralkyl” compounds such as M[CH2Si(CH3)3]4, M(CH2C6H5)4, and M[CH2C(CH3)3]4 (M = Ti, Zr, or Hf; Fig 2), were rationalized on the basis of the fact that unlike a compound having an ethyl ligand, th ...
Which is Aromatic?
... Predict the ratio of isomeric products A-C from the nitration of nitrobenzene, and comment on the rate of nitration of benzene compared to nitrobenzene. Rationalize your answers with resonance structures. ...
... Predict the ratio of isomeric products A-C from the nitration of nitrobenzene, and comment on the rate of nitration of benzene compared to nitrobenzene. Rationalize your answers with resonance structures. ...
Document
... To study the energy changes in reactions under conditions of constant volume, a “bomb calorimeter” (Fig. 6.6) is used. For a constant-volume process, the change in volume ΔV is equal to zero, so work (which is -PΔV) is also equal to zero. 歐亞書局 ...
... To study the energy changes in reactions under conditions of constant volume, a “bomb calorimeter” (Fig. 6.6) is used. For a constant-volume process, the change in volume ΔV is equal to zero, so work (which is -PΔV) is also equal to zero. 歐亞書局 ...
13 CHEMICAL EQUILIBRIUM W MODULE - 5
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...