Energy of Reactions
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
Chemistry 220
... Good morning! Do all the questions on this paper. You may use molecular models but no other aids (including calculators and cheat sheets) are permitted. The point values of the questions are shown, so budget your time accordingly. Make your answers as clear as possible. If you change an answer, indi ...
... Good morning! Do all the questions on this paper. You may use molecular models but no other aids (including calculators and cheat sheets) are permitted. The point values of the questions are shown, so budget your time accordingly. Make your answers as clear as possible. If you change an answer, indi ...
4th NOTES - Idaho State University
... heat capacity C = J/oC cal/oC In simple calorimeters, they are open to the air, so the rxn occurs at constant pressure Q = Qp = Hrxn (TOT) Usually want Hrxn on a per mol basis so divide by moles of the product formed or moles of the limiting reagent to get: More elaborate method of measuring heats ...
... heat capacity C = J/oC cal/oC In simple calorimeters, they are open to the air, so the rxn occurs at constant pressure Q = Qp = Hrxn (TOT) Usually want Hrxn on a per mol basis so divide by moles of the product formed or moles of the limiting reagent to get: More elaborate method of measuring heats ...
FINAL EXAM Spring 2012
... The last page of this examination is a periodic table [Gas constant = 8.314 J/mol K; 0.08206 L*atm/mole*K,1 faraday = 96500 J/V mol e-; at 25oC] 1) The reaction has the rate law, Rate = k[A][B]2. Which will cause the rate to increase the most? A) doubling [A] B) doubling [B] C) tripling [B] D) quadr ...
... The last page of this examination is a periodic table [Gas constant = 8.314 J/mol K; 0.08206 L*atm/mole*K,1 faraday = 96500 J/V mol e-; at 25oC] 1) The reaction has the rate law, Rate = k[A][B]2. Which will cause the rate to increase the most? A) doubling [A] B) doubling [B] C) tripling [B] D) quadr ...
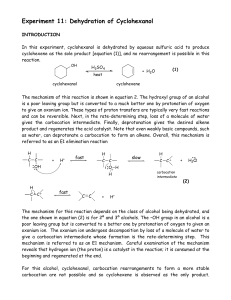
Dehydration of Cyclohexanol
... Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symmetrical ethers are the major products; these are formed by nucleophilic attack of an alcohol molecule on the oxonium ion formed from protonation of another alcohol molecu ...
... Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symmetrical ethers are the major products; these are formed by nucleophilic attack of an alcohol molecule on the oxonium ion formed from protonation of another alcohol molecu ...
Chapter 11 Chemical Reactions
... sulfide gas. Fe2S3 (s) + HCl (g) FeCl3 (s)+ H2S (g) Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. ...
... sulfide gas. Fe2S3 (s) + HCl (g) FeCl3 (s)+ H2S (g) Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. ...
CHM222A: Basic Physical Chemistry
... Ammonia production is the first stage of fertilizer preparation and the most difficult Global population and the nitrogen cycle, Vaclav Smil, Scientific American, July 1997 Fritz-Haber process replicated that of N-fixing bacteria and transformed the N-cycle Nitrogen compounds are also used in explos ...
... Ammonia production is the first stage of fertilizer preparation and the most difficult Global population and the nitrogen cycle, Vaclav Smil, Scientific American, July 1997 Fritz-Haber process replicated that of N-fixing bacteria and transformed the N-cycle Nitrogen compounds are also used in explos ...
Short Title PHYSICAL CHEMISTRY Full Title PHYSICAL
... Chemical equilibria: Phase Equilibria, chemical equilibrist in gases, reaction in solution. Factors affecting equilibrium, Le chateliers principle and calculations ...
... Chemical equilibria: Phase Equilibria, chemical equilibrist in gases, reaction in solution. Factors affecting equilibrium, Le chateliers principle and calculations ...
Worksheet - Venn Diagram Organic Chemistry ANSWER KEY
... Eastern Intermediate High School Honors Biology ...
... Eastern Intermediate High School Honors Biology ...
Lecture 2 - Chemistry at Winthrop University
... – They are due on the date posted on the class schedule ...
... – They are due on the date posted on the class schedule ...
Types of Changes in Matter
... reactants or products, but rather give a ratio we can use to compare ...
... reactants or products, but rather give a ratio we can use to compare ...