Fundamentals of Theoretical Organic Chemistry Lecture 1
... organic reactions are ionic, and ‘ionic’ frequently means bond dissociation to ion pairs. Clearly a bond which is already polar will undergo ionic dissociation with greater ease than a nonpolar bond, because a polarized bond is tending towards ionic dissociation. But the extent of bond polarization ...
... organic reactions are ionic, and ‘ionic’ frequently means bond dissociation to ion pairs. Clearly a bond which is already polar will undergo ionic dissociation with greater ease than a nonpolar bond, because a polarized bond is tending towards ionic dissociation. But the extent of bond polarization ...
102 Lab 7 Esters Fall05
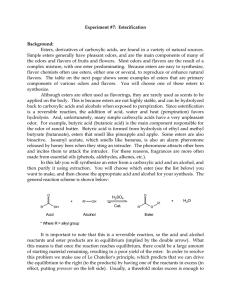
... reaction using a reflux apparatus (see figure below). Refluxing is a process where liquid (your reaction mixture) is heated to its boiling point, with a condenser attached, so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction ...
... reaction using a reflux apparatus (see figure below). Refluxing is a process where liquid (your reaction mixture) is heated to its boiling point, with a condenser attached, so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction ...
AP Chapter 5 Powerpoint
... cylinder with a movable piston. The cylinder is perfectly insulating, so no heat can escape to the surroundings. A spark initiates combustion of the butane, which forms carbon dioxide and water vapor. If we used this apparatus to measure the enthalpy change in the reaction, would the piston rise, fa ...
... cylinder with a movable piston. The cylinder is perfectly insulating, so no heat can escape to the surroundings. A spark initiates combustion of the butane, which forms carbon dioxide and water vapor. If we used this apparatus to measure the enthalpy change in the reaction, would the piston rise, fa ...
Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
delhi private school
... Q12. How will you bring about the following conversions in not more than two steps. (a) Propanone to Propene (b) Benzene to m-Nitroacetophenone (c) Chlorobenzene to benzoic acid (d) Propanoic acid to propene (e) Propyne to propan-2-ol 5 marks Q13. Explain the following with the help of suitable exam ...
... Q12. How will you bring about the following conversions in not more than two steps. (a) Propanone to Propene (b) Benzene to m-Nitroacetophenone (c) Chlorobenzene to benzoic acid (d) Propanoic acid to propene (e) Propyne to propan-2-ol 5 marks Q13. Explain the following with the help of suitable exam ...
Micellar Catalytic Effect of Cetyltrimethylammonium Bromide
... Chemistry acquired in 2011 from Gadjah Mada University, Yogyakarta, Indonesia. Since 2006 he was worked as researcher in Technical Implementation Unit for Development of Chemical Engineering Processes, Indonesian Institute of Sciences. In 2009 obtained a scholarship from Indonesian Institute of Scie ...
... Chemistry acquired in 2011 from Gadjah Mada University, Yogyakarta, Indonesia. Since 2006 he was worked as researcher in Technical Implementation Unit for Development of Chemical Engineering Processes, Indonesian Institute of Sciences. In 2009 obtained a scholarship from Indonesian Institute of Scie ...
Test
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 65 through 67 on the information and equation below. Antacids can be used to neutralize excess stomach acid. Brand A antacid contains the acidneutralizing agent magnesium hydroxide, Mg(OH)2. It ...
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 65 through 67 on the information and equation below. Antacids can be used to neutralize excess stomach acid. Brand A antacid contains the acidneutralizing agent magnesium hydroxide, Mg(OH)2. It ...
Higher Chemistry Learning Outcomes
... The modern Periodic Table is based on the work of Mendeleev who arranged the known elements in order of increasing atomic masses in conjunction with similar chemical properties, leaving gaps for undiscovered elements. There are variations in the densities, melting points and boiling points of th ...
... The modern Periodic Table is based on the work of Mendeleev who arranged the known elements in order of increasing atomic masses in conjunction with similar chemical properties, leaving gaps for undiscovered elements. There are variations in the densities, melting points and boiling points of th ...
- Solubility products -Thermochemistry
... • The heat of combustion is the heat released in the reaction of one mole of a substance in its standard state with oxygen. • The equation for the combustion of a compound must show the reaction of one mole of the compound with sufficient oxygen to convert all of the carbon and hydrogen present to g ...
... • The heat of combustion is the heat released in the reaction of one mole of a substance in its standard state with oxygen. • The equation for the combustion of a compound must show the reaction of one mole of the compound with sufficient oxygen to convert all of the carbon and hydrogen present to g ...
Description: This is an advanced placement course designed to
... Recommended Experiments With the introduction in 1999 of a required laboratory-based question on the free-response section of the AP Chemistry Exam, the inclusion of appropriate experiments into each AP Chemistry course is increasingly important….. It is unlikely that every student will complete al ...
... Recommended Experiments With the introduction in 1999 of a required laboratory-based question on the free-response section of the AP Chemistry Exam, the inclusion of appropriate experiments into each AP Chemistry course is increasingly important….. It is unlikely that every student will complete al ...
Standard Enthalpy of Formation
... measure changes in enthalpy, internal energy and entropies (ΔH, ΔU and ΔS, not the absolute values of U, H, and S, and we cannot tabulate absolute enthalpies of ...
... measure changes in enthalpy, internal energy and entropies (ΔH, ΔU and ΔS, not the absolute values of U, H, and S, and we cannot tabulate absolute enthalpies of ...
File
... (flame). A white, crumbly powder has properties that differ from the gas and metal. Wow! Mg melts at 650o, but MgO doesn’t melt until 2,800oC! ...
... (flame). A white, crumbly powder has properties that differ from the gas and metal. Wow! Mg melts at 650o, but MgO doesn’t melt until 2,800oC! ...