Electophilic Aromatic Substituion
... substituents ortho and para react with nucleophiles • Form addition intermediate (Meisenheimer complex) that is stabilized by electron-withdrawal • Halide ion is lost to give aromatic ring ...
... substituents ortho and para react with nucleophiles • Form addition intermediate (Meisenheimer complex) that is stabilized by electron-withdrawal • Halide ion is lost to give aromatic ring ...
Slides for lecture 7 - Aleksey Kocherzhenko
... Heat capacity depends on the conditions under which the substance is heated" (the reasons for this are discussed in detail in PChem II) " à Commonly reported are the molar heat capacity @ constant pressure,"Cp,m or @ constant volume, CV,m (or respective specific heat capacities)" Ø Heat capaciti ...
... Heat capacity depends on the conditions under which the substance is heated" (the reasons for this are discussed in detail in PChem II) " à Commonly reported are the molar heat capacity @ constant pressure,"Cp,m or @ constant volume, CV,m (or respective specific heat capacities)" Ø Heat capaciti ...
Chapter 18 lectures as pdf
... This chapter • Less basic sources of nucleophilic carbon • Formation of C-C bonds but some chance of reversibility • Strategies for control of reversibility • Use in synthesis • Related reactions ...
... This chapter • Less basic sources of nucleophilic carbon • Formation of C-C bonds but some chance of reversibility • Strategies for control of reversibility • Use in synthesis • Related reactions ...
2006 Practice Final Exam - Department of Chemistry | Oregon State
... Party—but just a little. Must save energy for Spring Break. Two words: Twinkies and TextMessaging. Spend some time thinking about those things 19 year olds think of… sex, parties, friends, music, reality TV, food, mutual funds, retirement plans, taking out the trash early, golf, effective denture cl ...
... Party—but just a little. Must save energy for Spring Break. Two words: Twinkies and TextMessaging. Spend some time thinking about those things 19 year olds think of… sex, parties, friends, music, reality TV, food, mutual funds, retirement plans, taking out the trash early, golf, effective denture cl ...
Chemical reactions
... When a chemical bond is broken, energy is absorbed, and when a chemical bond forms, energy is given off; thence, to initiate a reaction, some energy input is required (activation energy), and the exothermic or endothermic character of a reaction depends on the relative strength of broken bonds and f ...
... When a chemical bond is broken, energy is absorbed, and when a chemical bond forms, energy is given off; thence, to initiate a reaction, some energy input is required (activation energy), and the exothermic or endothermic character of a reaction depends on the relative strength of broken bonds and f ...
Syllabus - Chemistry
... Reaction Mechanism in coordination complexes and their spectral/magnetic Properties: Magnetic Properties and Electronic Spectra of Transition Metal Complexes Types of magnetic behaviour, magnetic susceptibility and magnetic moment; methods of determining magnetic susceptibility; spin-only formula; L ...
... Reaction Mechanism in coordination complexes and their spectral/magnetic Properties: Magnetic Properties and Electronic Spectra of Transition Metal Complexes Types of magnetic behaviour, magnetic susceptibility and magnetic moment; methods of determining magnetic susceptibility; spin-only formula; L ...
CHAPTER 9
... Most reactions are carried out in liquid solution or in the gaseous phase because in such situations a) activation energies are higher. b) it is easier for reactants to come in contact with each other. c) kinetic energies of reactants are lower. d) products are less apt to decompose. ...
... Most reactions are carried out in liquid solution or in the gaseous phase because in such situations a) activation energies are higher. b) it is easier for reactants to come in contact with each other. c) kinetic energies of reactants are lower. d) products are less apt to decompose. ...
Organic Halides
... Traditional usages distinguish ketal from acetals, where the acetal has one R group as H-. current accepted terminology classifies ketals as a subset of acetals. For engineering applications, “actal” is shorthand for the plastic polyoxymethylene, which is a polyacetal. Formation of an acteal occurs ...
... Traditional usages distinguish ketal from acetals, where the acetal has one R group as H-. current accepted terminology classifies ketals as a subset of acetals. For engineering applications, “actal” is shorthand for the plastic polyoxymethylene, which is a polyacetal. Formation of an acteal occurs ...
lecture10
... that could be produced by the system with not current flowing). The proportionality constant has two parts. Part (F, the Faraday constant) takes into account the fact that Gibbs free energy is on a per mole basis, while voltage is on a per coulomb basis and a mole is a different number than a coulom ...
... that could be produced by the system with not current flowing). The proportionality constant has two parts. Part (F, the Faraday constant) takes into account the fact that Gibbs free energy is on a per mole basis, while voltage is on a per coulomb basis and a mole is a different number than a coulom ...
Topics 10 and 20 Outline
... synthesis of new pathways and have considered the ethical and environmental implications of adopting green chemistry. (4.1, 4.5) Understandings: Nucleophilic Substitution Reactions: • SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular subst ...
... synthesis of new pathways and have considered the ethical and environmental implications of adopting green chemistry. (4.1, 4.5) Understandings: Nucleophilic Substitution Reactions: • SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular subst ...
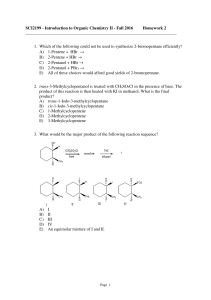
SCI2199 - Introduction to Organic Chemistry II
... 15. Which reagent(s) would transform propyl alcohol into propyl bromide? A) Concd. HBr and heat B) PBr3 C) NaBr/H2O and heat D) More than one of these choices. E) All of these choices. ...
... 15. Which reagent(s) would transform propyl alcohol into propyl bromide? A) Concd. HBr and heat B) PBr3 C) NaBr/H2O and heat D) More than one of these choices. E) All of these choices. ...
Chapter 5 Thermochemistry Energy :capacity to do work or to
... Heat is the energy transferred from a hotter object to a colder one. A combustion reaction, such as the burning of natural gas illustrated in Figure 5.1(b), releases the chemical energy stored in the molecules of the fuel. If we define the substances involved in the reaction as the system and every ...
... Heat is the energy transferred from a hotter object to a colder one. A combustion reaction, such as the burning of natural gas illustrated in Figure 5.1(b), releases the chemical energy stored in the molecules of the fuel. If we define the substances involved in the reaction as the system and every ...