Lecture syllabus - Linfield College
... compounds. After reviewing basic concepts from general chemistry, certain classes of compounds are studied in terms of ...
... compounds. After reviewing basic concepts from general chemistry, certain classes of compounds are studied in terms of ...
Fundamentals Of Organic Chemistry
... Fundamentals Of Organic Chemistry Part-4 (d) Dehydration removal of water ...
... Fundamentals Of Organic Chemistry Part-4 (d) Dehydration removal of water ...
Lecture 1 and 2a - Thermochemistry
... from the initial state and the final state. ΔE, however, is a state function, which means that the value of ΔE depends only on the initial and final states and not the path taken. ΔE can be calculated as Efinal – Einitial. Many chemical reactions and processes are carried out at constant pressure, s ...
... from the initial state and the final state. ΔE, however, is a state function, which means that the value of ΔE depends only on the initial and final states and not the path taken. ΔE can be calculated as Efinal – Einitial. Many chemical reactions and processes are carried out at constant pressure, s ...
course contents 160 - drseemaljelani
... science, and technology with a major in Chemistry. The Chemistry major enables you to view the world from a molecular perspective, and to solve complex problems that span the breadth of chemistry and other sciences. You can choose to specialize in Chemistry Through your studies you will gain transfe ...
... science, and technology with a major in Chemistry. The Chemistry major enables you to view the world from a molecular perspective, and to solve complex problems that span the breadth of chemistry and other sciences. You can choose to specialize in Chemistry Through your studies you will gain transfe ...
Chemical Kinetics
... Study at a point soon after they are mixed before product builds up. Reaction rate will depend only on concentration of the reactants. ...
... Study at a point soon after they are mixed before product builds up. Reaction rate will depend only on concentration of the reactants. ...
Entering and leaving group effects in Oh ligand substitutions
... The NH2- ligand stabilizes a lower (5-coordinate) transition state, because it is both a good sigma donor and a good π-donor. this accelerates rate-determining step: loss of Cl– ion Reaction studies of 16O/18O isotope effects indicate that OH– in the product complex comes from bulk H2O, not from OH– ...
... The NH2- ligand stabilizes a lower (5-coordinate) transition state, because it is both a good sigma donor and a good π-donor. this accelerates rate-determining step: loss of Cl– ion Reaction studies of 16O/18O isotope effects indicate that OH– in the product complex comes from bulk H2O, not from OH– ...
A-level Paper 1 Practice Paper 8 - A
... hydrochloric acid. All of the iron in the sample reacted, evolving hydrogen gas and forming a solution of iron(II) chloride. The volume of hydrogen evolved was 102 cm3, measured at 298 K and 110 kPa. The percentage, by mass, of iron in the sample can be determined using the volume of hydrogen ...
... hydrochloric acid. All of the iron in the sample reacted, evolving hydrogen gas and forming a solution of iron(II) chloride. The volume of hydrogen evolved was 102 cm3, measured at 298 K and 110 kPa. The percentage, by mass, of iron in the sample can be determined using the volume of hydrogen ...
BP208P. PHARMACEUTICAL ORGANIC CHEMISTRY
... 5. Melting point/Boiling point of organic compounds 6. Identification of the unknown compound from the literature using melting point/ boiling point. 7. Preparation of the derivatives and confirmation of the unknown compound by melting point/ boiling point. 8. Minimum 5 unknown organic compounds to ...
... 5. Melting point/Boiling point of organic compounds 6. Identification of the unknown compound from the literature using melting point/ boiling point. 7. Preparation of the derivatives and confirmation of the unknown compound by melting point/ boiling point. 8. Minimum 5 unknown organic compounds to ...
Gas and Thermo Notes
... Notice that the heat capacity is for a given object, while specific is for any object of that material, since we know how one gram will behave. The equations to calculate heat are: q = mc∆T ...
... Notice that the heat capacity is for a given object, while specific is for any object of that material, since we know how one gram will behave. The equations to calculate heat are: q = mc∆T ...
Lesson 3 Mechanisms of Organic Reactions
... and Br+), carbocations, or neutral molecules such as sulfur trioxide, SO3, or compounds of the general formula R-X, where X is an electron-withdrawing group. Nucleophiles are often, though not always, negatively charged. The most widely known nucleophiles are a hydroxide ion, alkoxide ions (RO-), th ...
... and Br+), carbocations, or neutral molecules such as sulfur trioxide, SO3, or compounds of the general formula R-X, where X is an electron-withdrawing group. Nucleophiles are often, though not always, negatively charged. The most widely known nucleophiles are a hydroxide ion, alkoxide ions (RO-), th ...
Chapter 4 Quantities of Reactants and Products 4.1 Chemical
... 4.7 Percent Composition and Empirical Formulas (p. 150) In a combustion analysis of a compound containing carbon and hydrogen, the compound reacts with oxygen and all of the carbon in the compound is converted to carbon dioxide and the hydrogen in the compound is converted to water. 2 C4H10(g) + 13 ...
... 4.7 Percent Composition and Empirical Formulas (p. 150) In a combustion analysis of a compound containing carbon and hydrogen, the compound reacts with oxygen and all of the carbon in the compound is converted to carbon dioxide and the hydrogen in the compound is converted to water. 2 C4H10(g) + 13 ...
Open questions (66 points total
... 2p 10 Give the electron formula of the ion formed in partial reaction 1, when a H+-ion is extracted from the chloroethane. In the electron formula, put the charge with the correct atom. For some monochloroalkanes, with at least 2 C-atoms per molecule, no alkene formation will take place during a r ...
... 2p 10 Give the electron formula of the ion formed in partial reaction 1, when a H+-ion is extracted from the chloroethane. In the electron formula, put the charge with the correct atom. For some monochloroalkanes, with at least 2 C-atoms per molecule, no alkene formation will take place during a r ...
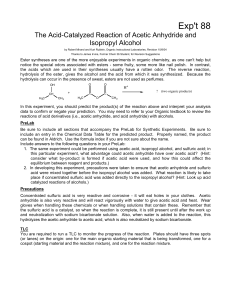
ESTERIFICATION
... reaction using a reflux apparatus (see figure below). Refluxing is a process where liquid (your reaction mixture) is heated to its boiling point, with a condenser attached, so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction ...
... reaction using a reflux apparatus (see figure below). Refluxing is a process where liquid (your reaction mixture) is heated to its boiling point, with a condenser attached, so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction ...
Spring Exam 4 - Chemistry
... Grading and Reporting: The examination scores will be posted in Blackboard as soon as possible after the examination. If an error has been made in scoring your answers, tell your instructor within 48 hours of the posting of your score. Be sure that your test has 60 questions, a periodic table, and t ...
... Grading and Reporting: The examination scores will be posted in Blackboard as soon as possible after the examination. If an error has been made in scoring your answers, tell your instructor within 48 hours of the posting of your score. Be sure that your test has 60 questions, a periodic table, and t ...
Chapter 6
... Stoichiometry deals with calculations about the masses of reactants and products involved in a chemical reaction. ...
... Stoichiometry deals with calculations about the masses of reactants and products involved in a chemical reaction. ...