june 2008 - The University of Sydney
... A string is attached to a wall. A lecturer holding the unattached end pulls with a constant force (parallel to the string) while moving his hand up and down (perpendicular to the string) to create a pulse travelling towards the wall. The lecturer now wants to produce a pulse that takes a longer time ...
... A string is attached to a wall. A lecturer holding the unattached end pulls with a constant force (parallel to the string) while moving his hand up and down (perpendicular to the string) to create a pulse travelling towards the wall. The lecturer now wants to produce a pulse that takes a longer time ...
Document
... • It is measure of heat change occurring during the process at constant temperature & volume. ...
... • It is measure of heat change occurring during the process at constant temperature & volume. ...
11 Thermodynamics and Thermochemistry
... The value of ∆G may be obtained by the formula below if ∆H, T, and ∆S is known: ∆G = ∆H - T∆S . When using this formula the temperature must be in Kelvin, and the units of energy for ∆H and ∆S must be the same. The relationship between ∆H, T, and ∆S as they relates to spontaneity is shown in ...
... The value of ∆G may be obtained by the formula below if ∆H, T, and ∆S is known: ∆G = ∆H - T∆S . When using this formula the temperature must be in Kelvin, and the units of energy for ∆H and ∆S must be the same. The relationship between ∆H, T, and ∆S as they relates to spontaneity is shown in ...
L1 - Internal Energy..
... Remember, a chemical system is described by P, V, n, T, and reactivity based on the properties of species reacting. Notice P and V are the variables of work. Also, notice that T, n (in terms of mass) and reactivity (in terms of specific heat) are the variables of heat transferred. ...
... Remember, a chemical system is described by P, V, n, T, and reactivity based on the properties of species reacting. Notice P and V are the variables of work. Also, notice that T, n (in terms of mass) and reactivity (in terms of specific heat) are the variables of heat transferred. ...
Week 11 - Guelph Physics
... Osmosis in biological cells (For now, we assume the cells are placed in solution of osmotically active solutes, which cannot diffuse through the cell membrane) The membrane of a biological cell is semi-permeable, and the cell’s interior (the lumen) contains osmotically active material. The lumen is ...
... Osmosis in biological cells (For now, we assume the cells are placed in solution of osmotically active solutes, which cannot diffuse through the cell membrane) The membrane of a biological cell is semi-permeable, and the cell’s interior (the lumen) contains osmotically active material. The lumen is ...
Chapter 3: The First Law of Thermodynamics for Closed Systems a
... system energy equation is in heat engine processes in which the system is approximated by an ideal gas, thus we will develop relations to determine the internal energy for an ideal gas. We will find also that a new property called Enthalpy will be useful both for Closed Systems and in particular for ...
... system energy equation is in heat engine processes in which the system is approximated by an ideal gas, thus we will develop relations to determine the internal energy for an ideal gas. We will find also that a new property called Enthalpy will be useful both for Closed Systems and in particular for ...
$doc.title
... "In all cases in which work is produced by the agency of heat, a quantity of heat is consumed which is proportional to the work done; and conversely, by the expenditure of an equal quantity of ...
... "In all cases in which work is produced by the agency of heat, a quantity of heat is consumed which is proportional to the work done; and conversely, by the expenditure of an equal quantity of ...
ASU Chain Reaction - Volume 2
... become warm. They reach a state called “thermal equilibrium.” Heat from the hot substance f lows into the cold substance until it is balanced between the two. As a result, both substances reach the same temperature. That temperature stays the same over time. If left alone, heat always f lows from ho ...
... become warm. They reach a state called “thermal equilibrium.” Heat from the hot substance f lows into the cold substance until it is balanced between the two. As a result, both substances reach the same temperature. That temperature stays the same over time. If left alone, heat always f lows from ho ...
1-3 - University of Reading
... work done on the gas goes into internal energy. This means there is no leakage of “heat”. • Such a compression (or expansion) where there is no flow of heat through the walls of the piston or vessel is called adiabatic. From the Greek a (not) dia (through) bainein (to go). • For generality with othe ...
... work done on the gas goes into internal energy. This means there is no leakage of “heat”. • Such a compression (or expansion) where there is no flow of heat through the walls of the piston or vessel is called adiabatic. From the Greek a (not) dia (through) bainein (to go). • For generality with othe ...
H 2 (g)
... supplied when the system is free to change its volume • some of the energy can return to the surroundings as expansion work ...
... supplied when the system is free to change its volume • some of the energy can return to the surroundings as expansion work ...
Ch. 6- Energetics
... A system does 50.2 J of work on its surroundings and there is a simultaneous 90.1 J heat transfer from the surroundings to the system. What is ΔEsystem? Work done on the surroundings by the system Heat transfers from the surroundings to the system wsystem = -50.2 J qsystem = +90.1 J ...
... A system does 50.2 J of work on its surroundings and there is a simultaneous 90.1 J heat transfer from the surroundings to the system. What is ΔEsystem? Work done on the surroundings by the system Heat transfers from the surroundings to the system wsystem = -50.2 J qsystem = +90.1 J ...
lecture1 - Unaab.edu.ng
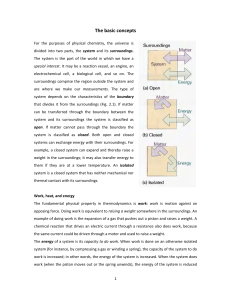
... Thermodynamics: This is the study of energy changes in chemical and physical processes and of the laws and relationships which govern them. Some terms are commonly used: (a) System; A system in thermodynamics is defined as collection of matter i.e that part of the universe under study. The rest of t ...
... Thermodynamics: This is the study of energy changes in chemical and physical processes and of the laws and relationships which govern them. Some terms are commonly used: (a) System; A system in thermodynamics is defined as collection of matter i.e that part of the universe under study. The rest of t ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.