Chapter 5 - Clayton State University
... Also known as Law of Conservation of Energy The total amount of energy in the universe is constant. ...
... Also known as Law of Conservation of Energy The total amount of energy in the universe is constant. ...
The Use and Misuse of the LUWS of Thermodynamics
... worse than nearly all the others in its field. Thermodynamics is incredibly badly presented, for the most part by people who do not understand it. The usual undergraduate course consists more of pretentious pseudo-philosophy than of anything relevant to experimental science. It is hardly surprising ...
... worse than nearly all the others in its field. Thermodynamics is incredibly badly presented, for the most part by people who do not understand it. The usual undergraduate course consists more of pretentious pseudo-philosophy than of anything relevant to experimental science. It is hardly surprising ...
Lecture 6 Free Energy
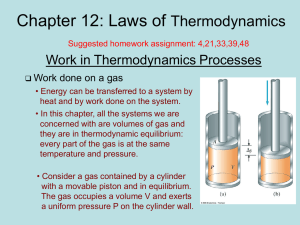
... of 300 K against the atmosphere pressure (1 atm) until equilibrium is reached. How much work is done by the system? ...
... of 300 K against the atmosphere pressure (1 atm) until equilibrium is reached. How much work is done by the system? ...
Thermodynamics
... the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. ...
... the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. ...
First law of thermodynamics
... 10.2.1 Deduce an expression for the work involved in a volume change of a gas at constant pressure. 10.2.2 State the first law of thermodynamics. 1 Students should be familiar with the terms system and surroundings. They should also appreciate that if a system and its surroundings are at different t ...
... 10.2.1 Deduce an expression for the work involved in a volume change of a gas at constant pressure. 10.2.2 State the first law of thermodynamics. 1 Students should be familiar with the terms system and surroundings. They should also appreciate that if a system and its surroundings are at different t ...
ppt
... Where Q is the heat given to the system by the surroundings which are at a an infinitesimally higher temperature, T, than the system ...
... Where Q is the heat given to the system by the surroundings which are at a an infinitesimally higher temperature, T, than the system ...
ourse 228 File
... (8) An air conditioner removes heat steadily from a house at a rate of 750Kj/min while consuming power at arate of 6 Kw. Determine the COP of the air conditioner and the rate of heat transfer from the room. (9) A house can be heated either by electric resistance heaters or by a heat pump. A househo ...
... (8) An air conditioner removes heat steadily from a house at a rate of 750Kj/min while consuming power at arate of 6 Kw. Determine the COP of the air conditioner and the rate of heat transfer from the room. (9) A house can be heated either by electric resistance heaters or by a heat pump. A househo ...
Word document format
... currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the system from the surroundings, q is positive. If one were carrying out the reaction i ...
... currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the system from the surroundings, q is positive. If one were carrying out the reaction i ...
UNIT I PART B 1). (i). A spherical balloon of diameter
... nature are irreversible processes. The natural process occurs due to the finite gradient between the two states of the system. For instance, heat flow between two bodies occurs due to the temperature gradient between the two bodies; this is in fact the natural flow of heat. Similarly, water flows fr ...
... nature are irreversible processes. The natural process occurs due to the finite gradient between the two states of the system. For instance, heat flow between two bodies occurs due to the temperature gradient between the two bodies; this is in fact the natural flow of heat. Similarly, water flows fr ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.