First Law of Thermodynamics {17}
... can be rewritten, with pV = nRT W nR(T2 T1 ) p203/4c17:3 ...
... can be rewritten, with pV = nRT W nR(T2 T1 ) p203/4c17:3 ...
chapter 5 lecture notes ppt
... When a 3.88 g sample of solid ammonium nitrate disolves in 60.0 g of water in a coffee cup calorimeter, the temperature drops from 23.0 °C to 18.4 °C. (a) Calculate H (in kJ/mol ammonium nitrate) for the solution process. Assume that the specific heat is constant and = 4.184 J/g°C. (b) Is this proc ...
... When a 3.88 g sample of solid ammonium nitrate disolves in 60.0 g of water in a coffee cup calorimeter, the temperature drops from 23.0 °C to 18.4 °C. (a) Calculate H (in kJ/mol ammonium nitrate) for the solution process. Assume that the specific heat is constant and = 4.184 J/g°C. (b) Is this proc ...
The Mayer-Joule Principle: The Foundation of
... blood. Although not a physicist by training, Mayer used existing data to arrive at a quantitative value for the exchange rate J that made heat and work comparable. Using modern notation and terminology, László Tisza15 describes Mayer’s argument. Suppose the energy needed to heat a mass m of dilute g ...
... blood. Although not a physicist by training, Mayer used existing data to arrive at a quantitative value for the exchange rate J that made heat and work comparable. Using modern notation and terminology, László Tisza15 describes Mayer’s argument. Suppose the energy needed to heat a mass m of dilute g ...
Experimental Enthalpy of Fusion and Heat Capacity
... As already indicated, the only other experimental heat capacity data have been obtained by adiabatic calorimetry [12] but, where the comparison is possible (300-350 K), they differ substantially (by about 8%) from ours. It should be stressed that the thermodynamic evaluation performed by Pankratz [1 ...
... As already indicated, the only other experimental heat capacity data have been obtained by adiabatic calorimetry [12] but, where the comparison is possible (300-350 K), they differ substantially (by about 8%) from ours. It should be stressed that the thermodynamic evaluation performed by Pankratz [1 ...
國立台北科技大學 冷凍與空調工程研究所碩士在職專班
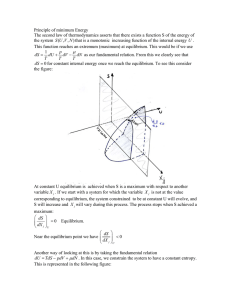
... When a process proceeds in such a manner that the system remains infinitesimally close to an equilibrium state at all times, it is called a quasi-static, or quasiequilibrium, process. (Fig. 1-29) ...
... When a process proceeds in such a manner that the system remains infinitesimally close to an equilibrium state at all times, it is called a quasi-static, or quasiequilibrium, process. (Fig. 1-29) ...
Lecture 3: FIRST LAW OF THERMODYNAMICS
... If a THERMALLY ISOLATED system is brought from one equilibrium state to another, the work necessary is independent of the process used. Joule’s observation “independent of the process used” means. there is a state function U, which we call the internal energy. ...
... If a THERMALLY ISOLATED system is brought from one equilibrium state to another, the work necessary is independent of the process used. Joule’s observation “independent of the process used” means. there is a state function U, which we call the internal energy. ...
AP Physics – Second Law of Thermodynamics
... The argument goes like this: the universe must go from an ordered state to a disordered state according to the second law of thermodynamics. Yet for life to have evolved as Darwin said it has would require that life have gone from a low state of order to a higher state of order. This is clearly proh ...
... The argument goes like this: the universe must go from an ordered state to a disordered state according to the second law of thermodynamics. Yet for life to have evolved as Darwin said it has would require that life have gone from a low state of order to a higher state of order. This is clearly proh ...
Chapter 16 - Faculty Server Contact
... efficiency that is better than the efficiency of a reversible engine • In practice, all heat engines will always be irreversible to some extent • Carnot’s result sets an absolute limit on the efficiency of all real heat engines ...
... efficiency that is better than the efficiency of a reversible engine • In practice, all heat engines will always be irreversible to some extent • Carnot’s result sets an absolute limit on the efficiency of all real heat engines ...
Fluids – Lecture 11 Notes
... Definition and implications A compressible flow is a flow in which the fluid density ρ varies significantly within the flowfield. Therefore, ρ(x, y, z) must now be treated as a field variable rather than simply a constant. Typically, significant density variations start to appear when the flow Mach number exc ...
... Definition and implications A compressible flow is a flow in which the fluid density ρ varies significantly within the flowfield. Therefore, ρ(x, y, z) must now be treated as a field variable rather than simply a constant. Typically, significant density variations start to appear when the flow Mach number exc ...
work
... It is an experimental fact that the work done during these two different processes is the same. The total work is the same in all adiabatic processes between any two equilibrium states having the same kinetic and potential energy!!! This is ...
... It is an experimental fact that the work done during these two different processes is the same. The total work is the same in all adiabatic processes between any two equilibrium states having the same kinetic and potential energy!!! This is ...
Consequences of the relation between temperature, heat, and
... which water freezes as 0 degrees. Last time, we introduced the absolute (Kelvin) temperature scale- a scale with a zero point and no possibility of a lower temperature- without much discussion of where it comes from. What ...
... which water freezes as 0 degrees. Last time, we introduced the absolute (Kelvin) temperature scale- a scale with a zero point and no possibility of a lower temperature- without much discussion of where it comes from. What ...
ESO201A: Thermodynamics
... nozzle, detailed analysis of a compressor, comparison of reversible adiabatic and reversible isothermal processes to get an ideal benchmarking process for a compressor, isentropic efficiency of a compressor, use of intercooler in intermediate stages of compression to achieve nearly isothermal proces ...
... nozzle, detailed analysis of a compressor, comparison of reversible adiabatic and reversible isothermal processes to get an ideal benchmarking process for a compressor, isentropic efficiency of a compressor, use of intercooler in intermediate stages of compression to achieve nearly isothermal proces ...
Chapter 5 Thermochemistry Student Outline Notes File
... -- ____________ the heat capacity the more heat required to raise a rise in temperature. Molar heat capacity = the heat capacity of 1 __________ of a substance Specific heat = heat capacity of 1 _________ of a substance q = _____________________________________________________(formula) a couple of ...
... -- ____________ the heat capacity the more heat required to raise a rise in temperature. Molar heat capacity = the heat capacity of 1 __________ of a substance Specific heat = heat capacity of 1 _________ of a substance q = _____________________________________________________(formula) a couple of ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.