Ppt19(PS8)_Thermo_Hess
... • Note: if P is NOT constant during some process, the amount of heat (flow) is NOT equal to the change in H (DHsys)! “H” is “enthalpy”, NOT heat! The change in H just happens to equal q when a process is carried out at constant P. Ppt19 ...
... • Note: if P is NOT constant during some process, the amount of heat (flow) is NOT equal to the change in H (DHsys)! “H” is “enthalpy”, NOT heat! The change in H just happens to equal q when a process is carried out at constant P. Ppt19 ...
Thermodynamics
... energy which really means that the total Energy of the universe remains constant! Second Law of Thermodynamics--The entropy [)S--I know, the word entropy has no “S” in it--I don’t decide these things! It’s just a fancy word for disorder or chaos.] of the universe is always increasing. [Think about a ...
... energy which really means that the total Energy of the universe remains constant! Second Law of Thermodynamics--The entropy [)S--I know, the word entropy has no “S” in it--I don’t decide these things! It’s just a fancy word for disorder or chaos.] of the universe is always increasing. [Think about a ...
Carnot - UniMAP Portal
... a system is in steady state then the recently observed behaviors of the system will continue into the future. In stochastic systems, the probabilities that various different states will be repeated will remain constant. • In many systems, steady state is not achieved until some time has elapsed afte ...
... a system is in steady state then the recently observed behaviors of the system will continue into the future. In stochastic systems, the probabilities that various different states will be repeated will remain constant. • In many systems, steady state is not achieved until some time has elapsed afte ...
Theoretische Physik IV: Statistische Mechanik, Exercise 6
... and isothermal compressions. Why such a substance cannot exist? ...
... and isothermal compressions. Why such a substance cannot exist? ...
Lecture 1
... words the change in entropy adds to zero over the entire cycle. The net entropy change per cycle: S SL SH 0 (In a Carnot engine there are two reversible energy transfers as heat, and thus two changes in the entropy of the working substance - one at temperature TH and one at TL.) Entropy as ...
... words the change in entropy adds to zero over the entire cycle. The net entropy change per cycle: S SL SH 0 (In a Carnot engine there are two reversible energy transfers as heat, and thus two changes in the entropy of the working substance - one at temperature TH and one at TL.) Entropy as ...
PHYS2LessonsContinued
... upward force on the lid and moves it through a displacement. The state of popcorn changes in this process, since the volume, temperature, and pressure of the popcorn all changes as it pops. The said process, in which there are changes in the state of thermodynamics system, is called thermodynamics p ...
... upward force on the lid and moves it through a displacement. The state of popcorn changes in this process, since the volume, temperature, and pressure of the popcorn all changes as it pops. The said process, in which there are changes in the state of thermodynamics system, is called thermodynamics p ...
Final exam questions for Chemical Engineer BSc
... 20. Conductivity of electrolytes, ionic motion. Conductance and conductivity of electrolyte solutions. Measurement of conductivity. Molar conductivity and its dependence on concentration: weak and strong electrolytes. The Kohlrausch law of the independent migration of ions. Ionic motion in electric ...
... 20. Conductivity of electrolytes, ionic motion. Conductance and conductivity of electrolyte solutions. Measurement of conductivity. Molar conductivity and its dependence on concentration: weak and strong electrolytes. The Kohlrausch law of the independent migration of ions. Ionic motion in electric ...
Kémiai technológia I
... 20. Conductivity of electrolytes, ionic motion. Conductance and conductivity of electrolyte solutions. Measurement of conductivity. Molar conductivity and its dependence on concentration: weak and strong electrolytes. The Kohlrausch law of the independent migration of ions. Ionic motion in electric ...
... 20. Conductivity of electrolytes, ionic motion. Conductance and conductivity of electrolyte solutions. Measurement of conductivity. Molar conductivity and its dependence on concentration: weak and strong electrolytes. The Kohlrausch law of the independent migration of ions. Ionic motion in electric ...
Document
... An amount of heat equal to 2500 J is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? A. ...
... An amount of heat equal to 2500 J is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? A. ...
7. Heat capacity
... When C is large > a given amount of heating results in only a small temperature rise (the system has a large capacity for heat) The heat capacity depends on conditions: - system constrained to have constant volume > Cv (heat capacity at constant volume, or isochoric heat capacity) - system subject t ...
... When C is large > a given amount of heating results in only a small temperature rise (the system has a large capacity for heat) The heat capacity depends on conditions: - system constrained to have constant volume > Cv (heat capacity at constant volume, or isochoric heat capacity) - system subject t ...
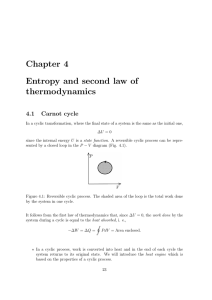
Chapter 4 Entropy and second law of thermodynamics
... the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behind and besides him: there is only one possibility (multiplicity is very low). Looking now ...
... the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behind and besides him: there is only one possibility (multiplicity is very low). Looking now ...
The state of a simple compressible system is completely specified by
... any of these must be considered, then one additional property must be known for each added effect. Example: for motion (kinetic) effects, velocity must be known. “Compressible” = volume not fixed. “Independent” = one property of a pair can be changed without affecting the other. Example: a. temperat ...
... any of these must be considered, then one additional property must be known for each added effect. Example: for motion (kinetic) effects, velocity must be known. “Compressible” = volume not fixed. “Independent” = one property of a pair can be changed without affecting the other. Example: a. temperat ...
A-Basic on Thermal Management
... Convection is the transfer of heat energy between a surface and a moving fluid at different temperatures. The convection is comprosed by two mechanisms. In addition to energy transfer due to random molecular motion , energy is also transferred by the bulk (or macroscopic) motion of the fluid. When n ...
... Convection is the transfer of heat energy between a surface and a moving fluid at different temperatures. The convection is comprosed by two mechanisms. In addition to energy transfer due to random molecular motion , energy is also transferred by the bulk (or macroscopic) motion of the fluid. When n ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.