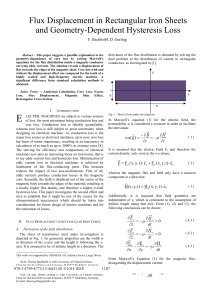
Ed 713.22 Magnet Power Point Presentation 2.1
... magnetic fields in three dimensions. They are defined as follows. If at any point on such a line we place an ideal compass needle, free to turn in any direction (unlike the usual compass needle, which stays horizontal) then the needle will always point along the field line. Field lines converge wher ...
... magnetic fields in three dimensions. They are defined as follows. If at any point on such a line we place an ideal compass needle, free to turn in any direction (unlike the usual compass needle, which stays horizontal) then the needle will always point along the field line. Field lines converge wher ...
Electricity & Magnetism Review 4: Units 17-19, 22-23
... magnetic field B. Calculate the induced emf in the coil. The flux through the loop at any time t is: B = NBA cos q = NBA cos wt ...
... magnetic field B. Calculate the induced emf in the coil. The flux through the loop at any time t is: B = NBA cos q = NBA cos wt ...
Z-pinch
... surface - averaged magnetic pressure (at the coils) – For configurations like the tokamak the maximum field at the magnet is of order twice the field in the plasma, whereas in the RFP the field at the magnet is less than in the plasma. – The engineering beta in an RFP reactor might be as much as twi ...
... surface - averaged magnetic pressure (at the coils) – For configurations like the tokamak the maximum field at the magnet is of order twice the field in the plasma, whereas in the RFP the field at the magnet is less than in the plasma. – The engineering beta in an RFP reactor might be as much as twi ...
CHAPTER- 1 : FUNDAMENTALS OF MAGETIC
... circuit is zero. Parallel Magnetic Circuit : Consider a parallel magnetic circuit shown in figure 4. Coil carrying a current I produces flux in path EFAB. At point B. flux gets divided into two paths, and such that ...
... circuit is zero. Parallel Magnetic Circuit : Consider a parallel magnetic circuit shown in figure 4. Coil carrying a current I produces flux in path EFAB. At point B. flux gets divided into two paths, and such that ...
electromagnetic induction
... Electromagnetic Induction, continued • The magnetic force acts on moving electric charges. – The force is at its maximum value when the charge moves perpendicularly to the field. – As the angle between the charge’s direction and the direction of the magnetic field decreases, the force on the charge ...
... Electromagnetic Induction, continued • The magnetic force acts on moving electric charges. – The force is at its maximum value when the charge moves perpendicularly to the field. – As the angle between the charge’s direction and the direction of the magnetic field decreases, the force on the charge ...
Electricity Ch. 18 Sect. 3
... Electromagnetic Induction, continued • The magnetic force acts on moving electric charges. – The force is at its maximum value when the charge moves perpendicularly to the field. – As the angle between the charge’s direction and the direction of the magnetic field decreases, the force on the charge ...
... Electromagnetic Induction, continued • The magnetic force acts on moving electric charges. – The force is at its maximum value when the charge moves perpendicularly to the field. – As the angle between the charge’s direction and the direction of the magnetic field decreases, the force on the charge ...
induced current
... Since a source emf is always needed to produce a current, the coil behaves as if it were a source of emf. This emf is known as the induced emf. ...
... Since a source emf is always needed to produce a current, the coil behaves as if it were a source of emf. This emf is known as the induced emf. ...
The Study of Cavitation Bubble- Surface Plasmon Resonance
... Water is unique among molecules as it is full of hydrogen bonds. The hydrogen bonds in water make water more colorless than gas. The role of hydrogen bonding can be determined by comparing water in its gaseous and liquid phase. When comparing the vibrational transitions between gaseous and liquid wa ...
... Water is unique among molecules as it is full of hydrogen bonds. The hydrogen bonds in water make water more colorless than gas. The role of hydrogen bonding can be determined by comparing water in its gaseous and liquid phase. When comparing the vibrational transitions between gaseous and liquid wa ...
Magnetochemistry

Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.