Homework 7
... of B = 0.390 T. Calculate the magnitude of the magnetic force on the wire assuming that the angle between the magnetic field and the current is (a) θa = 60.0◦ , (b) θb = 90.0◦ , and (c) θc = 120◦ . Using our formula for the force on a wire due to a uniform field we have F = Il × B ...
... of B = 0.390 T. Calculate the magnitude of the magnetic force on the wire assuming that the angle between the magnetic field and the current is (a) θa = 60.0◦ , (b) θb = 90.0◦ , and (c) θc = 120◦ . Using our formula for the force on a wire due to a uniform field we have F = Il × B ...
Formulas and constants Mass of electron m = 9.1. 10 kg
... Formulas and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...
... Formulas and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...
Crystal Field Theory
... ligands are varied along the series. Ligands that give rise to high energy transition (such as CO) is referred to as a strong-field ligand. Ligands that give rise to low energy transitions (such as Br-) referred to as weak-field ligand. Magnetic measurements Used to determine the number of unpaired ...
... ligands are varied along the series. Ligands that give rise to high energy transition (such as CO) is referred to as a strong-field ligand. Ligands that give rise to low energy transitions (such as Br-) referred to as weak-field ligand. Magnetic measurements Used to determine the number of unpaired ...
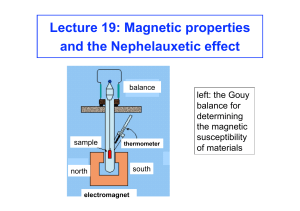
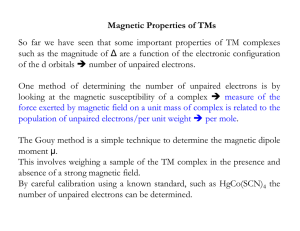
Magnetic Properties of TMs So far we have seen that some
... This is a case that involves a spin crossover for the d6 Fe(II) ion. The crossover involves going from high spin S = 2 (t2g4eg2) to low spin S ...
... This is a case that involves a spin crossover for the d6 Fe(II) ion. The crossover involves going from high spin S = 2 (t2g4eg2) to low spin S ...
Magnetochemistry

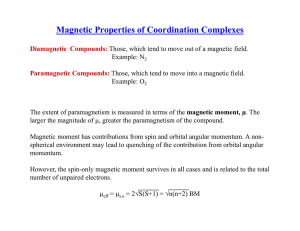
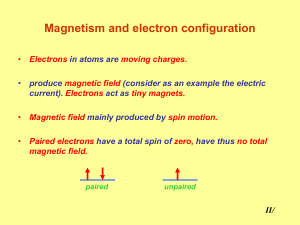
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.