Name Date Class 4.1 Follow Along Notes – Review Questions. How
... Dalton studied the _______________ in which elements combine in __________________ reactions. He observed that when atoms ___________, they maintain their own _________________ unless they combine in a chemical reaction. How was Democritus’s idea of the atom different from Dalton’s? Democritus’s ide ...
... Dalton studied the _______________ in which elements combine in __________________ reactions. He observed that when atoms ___________, they maintain their own _________________ unless they combine in a chemical reaction. How was Democritus’s idea of the atom different from Dalton’s? Democritus’s ide ...
CHEM 101 - Virginia State University
... • Mass: while the SI Unit is the kg… we usually report mass in grams. • Volume: The derived unit of Volume is the m3 yet in measurements we vary the unit depending on the substance… liquids are usually measured in mL, gases in L – 1000L=1m3 and 1mL=1cm3 ...
... • Mass: while the SI Unit is the kg… we usually report mass in grams. • Volume: The derived unit of Volume is the m3 yet in measurements we vary the unit depending on the substance… liquids are usually measured in mL, gases in L – 1000L=1m3 and 1mL=1cm3 ...
(halogens group) 4-write down the electronic configuration
... 5- the element with the same physical and chemical properties have been put in horizontal periods. In vertical group ...
... 5- the element with the same physical and chemical properties have been put in horizontal periods. In vertical group ...
Etymology of Atom - New Academic Science
... Leucippus of Miletus is thought to have originated the atomic philosophy. His famous disciple, Democritus of Abdera, named the building blocks of matter. He believed that atoms were uniform, solid, hard, incompressible, and indestructible and keep moving in empty space in infinite numbers till stopp ...
... Leucippus of Miletus is thought to have originated the atomic philosophy. His famous disciple, Democritus of Abdera, named the building blocks of matter. He believed that atoms were uniform, solid, hard, incompressible, and indestructible and keep moving in empty space in infinite numbers till stopp ...
atom
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
Inorganic Chemistry/Chemical Bonding/Lewis Dot Structures
... Once all lone pairs are placed, atoms, especially the central atoms, may not have an octet of electrons. In this case, the atoms must form a double bond; a lone pair of electrons is moved to form a second bond between the two atoms. As the bonding pair is shared between the two atoms, the atom that ...
... Once all lone pairs are placed, atoms, especially the central atoms, may not have an octet of electrons. In this case, the atoms must form a double bond; a lone pair of electrons is moved to form a second bond between the two atoms. As the bonding pair is shared between the two atoms, the atom that ...
Ch 2 ppt - Houston ISD
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
Ch 6 PPT - Blountstown Middle School
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
CS3_Ch 6 - Leon County Schools
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
atomic theory - chemistryatdulwich
... approximately one hundred thousand times greater than the radius of the nucleus. The nucleus has a very high density. This is because the protons, which all have ‘like’ charges are drawn very close together by very powerful nuclear forces. These forces need to be powerful as they Topic2atomictheory ...
... approximately one hundred thousand times greater than the radius of the nucleus. The nucleus has a very high density. This is because the protons, which all have ‘like’ charges are drawn very close together by very powerful nuclear forces. These forces need to be powerful as they Topic2atomictheory ...
Boron Group Compounds Oxidation States Boron
... Indium is more commonly found in the +1 oxidation state, while thallium is only found in this state (e.g. TlBr) This behavior is also seen in other p-block groupings, and is explained by the inert pair effect (results from the ionization energies of the 2nd and 3rd electrons in period 4 and heavier ...
... Indium is more commonly found in the +1 oxidation state, while thallium is only found in this state (e.g. TlBr) This behavior is also seen in other p-block groupings, and is explained by the inert pair effect (results from the ionization energies of the 2nd and 3rd electrons in period 4 and heavier ...
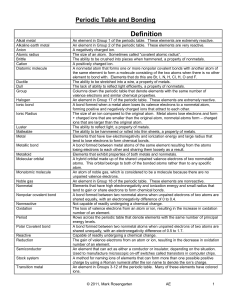
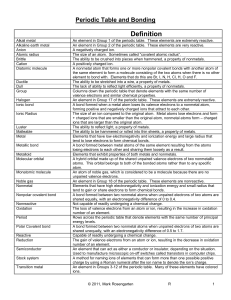
Unit 6: Periodic Table and Bonding
... the same element to form a molecule consisting of the two atoms when there is no other element to bond with. Elements that do this are Br, I, N, H, Cl, H, O and F. The ability to be stretched into a wire, a property of metals. The lack of ability to reflect light efficiently, a property of nonmetals ...
... the same element to form a molecule consisting of the two atoms when there is no other element to bond with. Elements that do this are Br, I, N, H, Cl, H, O and F. The ability to be stretched into a wire, a property of metals. The lack of ability to reflect light efficiently, a property of nonmetals ...
2. Building a new atomic model from scratch
... that does make mechanical sense, we could start with just a proton and an electron. We could also make the assumption that the proton and electron have a definite size and possesses a non-zero radius. We could also assume that the proton and electron are attracted to each other by the electrostatic ...
... that does make mechanical sense, we could start with just a proton and an electron. We could also make the assumption that the proton and electron have a definite size and possesses a non-zero radius. We could also assume that the proton and electron are attracted to each other by the electrostatic ...
Lesson 1
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
... • An atom is a building block of matter. An element is matter made of only one type of atom. A compound is a substance that contains two or more elements. • A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that ...
Isotopes
... calculations easier. It’s kind of like “mole.” We made up these units to make the numbers make more sense. ◦ 1 amu = 1/12th the mass of a Carbon-12 atom ...
... calculations easier. It’s kind of like “mole.” We made up these units to make the numbers make more sense. ◦ 1 amu = 1/12th the mass of a Carbon-12 atom ...
atom
... • The atomic number of an atom is given by its number of protons. The mass number of an atom is the sum of its neutrons and protons. atomic number = number of protons = number of electrons mass number = atomic number + number of neutrons ...
... • The atomic number of an atom is given by its number of protons. The mass number of an atom is the sum of its neutrons and protons. atomic number = number of protons = number of electrons mass number = atomic number + number of neutrons ...
atomic number
... completely wrong! Who am I? 4. I may be small, but I’m a lot heavier than those electrons.Maybe that’s why they orbit around me! What am I? 5. I’m using electrons to study what is inside protons. Who am I and where do I work? 6. I am one of these: a molecule, a proton, an electron, an atom or a nucl ...
... completely wrong! Who am I? 4. I may be small, but I’m a lot heavier than those electrons.Maybe that’s why they orbit around me! What am I? 5. I’m using electrons to study what is inside protons. Who am I and where do I work? 6. I am one of these: a molecule, a proton, an electron, an atom or a nucl ...
File
... A) Daltons Theory Of Atomic Mass B) Law of Chemistry C) Symbols of an Element D) Average Mass of an Isotope ...
... A) Daltons Theory Of Atomic Mass B) Law of Chemistry C) Symbols of an Element D) Average Mass of an Isotope ...
Atomic Structure - Monona Grove School District
... At the beginning of the last century, Dr. Wilhelm Fliess noticed identical rhythms in the case histories of his patients. He observed active and passive phases in the physical, emotional and mental aspects of humans. From these observations he derived the principle of the biorhythms, which state tha ...
... At the beginning of the last century, Dr. Wilhelm Fliess noticed identical rhythms in the case histories of his patients. He observed active and passive phases in the physical, emotional and mental aspects of humans. From these observations he derived the principle of the biorhythms, which state tha ...
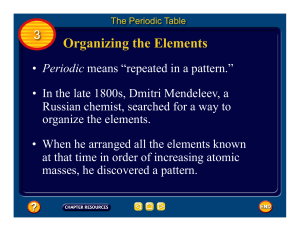
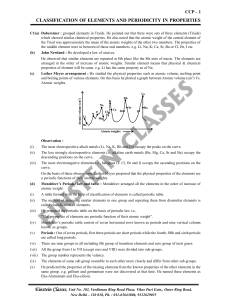
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN
... A modern version of table contains horizontal rows known as periods (which Mendeleev called series). Elements having similar outer electronic configuration in their atoms are grouped in vertical columns; these are referred as Groups or Families. ...
... A modern version of table contains horizontal rows known as periods (which Mendeleev called series). Elements having similar outer electronic configuration in their atoms are grouped in vertical columns; these are referred as Groups or Families. ...
Chemistry of Uniqueness and Value
... ● Chemistry is the study of turning stuff into other stuff and identifying the composition and components of that stuff. While we can do many amazing things with chemistry, there are limits on what we can do on earth and on what we can do as humans in our universe. ● Chemistry and other science ...
... ● Chemistry is the study of turning stuff into other stuff and identifying the composition and components of that stuff. While we can do many amazing things with chemistry, there are limits on what we can do on earth and on what we can do as humans in our universe. ● Chemistry and other science ...
Chapter 2
... (2) Law of conservation of mass The total masses of material present before and after a chemical reaction are identical [postulate 3] (3) Law of multiple proportions If elements A & B combine to form more than one compound, the masses of B which can combine with a given mass of A are in the rati ...
... (2) Law of conservation of mass The total masses of material present before and after a chemical reaction are identical [postulate 3] (3) Law of multiple proportions If elements A & B combine to form more than one compound, the masses of B which can combine with a given mass of A are in the rati ...