Dalton`s Personal Life
... • The Planck law gives the intensity radiated by a blackbody as a function of frequency (or wavelength). • Planck was also a philosopher of science. • In his Scientific Autobiography and Other Papers, he stated Planck's Principle, which holds that – "A new scientific truth does not triumph by convi ...
... • The Planck law gives the intensity radiated by a blackbody as a function of frequency (or wavelength). • Planck was also a philosopher of science. • In his Scientific Autobiography and Other Papers, he stated Planck's Principle, which holds that – "A new scientific truth does not triumph by convi ...
10/9 atomic structure powerpoint 2
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
Chapter 4
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
... Magnesium has three isotopes. 78.99% magnesium 24 with a mass of 23.9850 amu, 10.00% magnesium 25 with a mass of 24.9858 amu, and the rest magnesium 26 with a mass of 25.9826 amu. What is the atomic mass of magnesium? If not told otherwise, the mass of the isotope is the mass number in amu ...
1 Which of the following has the least mass
... C isotope D quark 2 Which of the following has the least mass in an atom? A neutron B proton C electron D nucleus 3 Which of the following showed electrons in specific, fixed orbits? A Rutherford Model B Planck Model C Bohr Model D Quantum Model 4 An atom that is electrically neutral must contain A ...
... C isotope D quark 2 Which of the following has the least mass in an atom? A neutron B proton C electron D nucleus 3 Which of the following showed electrons in specific, fixed orbits? A Rutherford Model B Planck Model C Bohr Model D Quantum Model 4 An atom that is electrically neutral must contain A ...
drawing bohr models
... Bohr’s Atomic Theory Ex.3) Draw a Bohr model of Lithium-3. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the she ...
... Bohr’s Atomic Theory Ex.3) Draw a Bohr model of Lithium-3. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the she ...
Activity 4 Are Atoms Indivisible?
... same evidence, H. Nagaoka, a Japanese scientist, modeled the atom as a large positively charged sphere surrounded by a ring of negative electrons. Rutherford’s Discovery of the Nucleus For several years there was no evidence to contradict either Thomson’s or Nagaoka’s atomic models. However, in the ...
... same evidence, H. Nagaoka, a Japanese scientist, modeled the atom as a large positively charged sphere surrounded by a ring of negative electrons. Rutherford’s Discovery of the Nucleus For several years there was no evidence to contradict either Thomson’s or Nagaoka’s atomic models. However, in the ...
- Angelo State University
... of the volume of the atom was empty space, through which the electrons were dispersed in some fashion. • The positively charged particles within the nucleus are called protons; there must be one electron for each proton for an atom to be electrically neutral. • This did not account for all of the ma ...
... of the volume of the atom was empty space, through which the electrons were dispersed in some fashion. • The positively charged particles within the nucleus are called protons; there must be one electron for each proton for an atom to be electrically neutral. • This did not account for all of the ma ...
Atoms and Molecules
... properties. Mendeleyev was able to organize the table in its present form even though many of the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as ne ...
... properties. Mendeleyev was able to organize the table in its present form even though many of the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as ne ...
Student Ch 11 Electrons-1
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
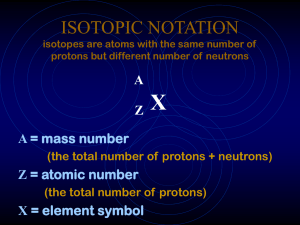
ISOTOPIC NOTATION isotopes are atoms with the same number
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
weighted average atomic mass
... • Find out the names that 110, 111, 112, 114, and 116 have now been given. What is the latest news about element 118? • Who/what makes the decisions about element names? • How long does it take for a name to be decided upon? • Record your source(s) using MLA formatting. Read your notes about Chromat ...
... • Find out the names that 110, 111, 112, 114, and 116 have now been given. What is the latest news about element 118? • Who/what makes the decisions about element names? • How long does it take for a name to be decided upon? • Record your source(s) using MLA formatting. Read your notes about Chromat ...
Structure of the Atom
... experiment is carried out using a foil of a metal other than gold? Answer: If the α-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin f ...
... experiment is carried out using a foil of a metal other than gold? Answer: If the α-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin f ...
GLUCOSE - npd117.net
... compounds are usually different from the elements that form it Ex.Hydrogen (H) and Oxygen (O) are gasses ...
... compounds are usually different from the elements that form it Ex.Hydrogen (H) and Oxygen (O) are gasses ...
Science 1206 - Naming and Writing Formulas for Chemical
... Third Level (Outermost) __________________ ...
... Third Level (Outermost) __________________ ...
2.1 Introduction
... matter can be divided infinitely. Both claims were based not on evidence but on visionary belief: one in unity, the other in diversity. In Section 2.3 we will discuss the evidence for the existence of atoms, but first we need to look at the diverse forms of matter. ...
... matter can be divided infinitely. Both claims were based not on evidence but on visionary belief: one in unity, the other in diversity. In Section 2.3 we will discuss the evidence for the existence of atoms, but first we need to look at the diverse forms of matter. ...
Bonding - Inorganic Chemistry
... 2 Hybridisation: Hybridisation can also affect the covalent radius since s orbitals are more contracted than p orbitals. The radius will decrease with an increase in the s character. C(sp3) 0.77 Å ; C(sp2) 0.73 Å ; C(sp) 0.70 Å. 3 Electronegativities: When there is great difference in electronegativ ...
... 2 Hybridisation: Hybridisation can also affect the covalent radius since s orbitals are more contracted than p orbitals. The radius will decrease with an increase in the s character. C(sp3) 0.77 Å ; C(sp2) 0.73 Å ; C(sp) 0.70 Å. 3 Electronegativities: When there is great difference in electronegativ ...
Atoms and Molecules
... Q: How many elements are there? A: There are 117 known elements. 90 of them are naturally occurring elements, and scientists have been able to create 27 more in the laboratory. Note: In 1869, Dmitri Mendeleyev was credited with putting together the Periodic Table of Elements. He listed all of the kn ...
... Q: How many elements are there? A: There are 117 known elements. 90 of them are naturally occurring elements, and scientists have been able to create 27 more in the laboratory. Note: In 1869, Dmitri Mendeleyev was credited with putting together the Periodic Table of Elements. He listed all of the kn ...
Module 3: Physical Science
... Elements are the simplest forms of matter. They can exist alone or in various combinations. Different elements can chemically combine to form molecules or molecular compounds. For example, water is a compound, consisting of water molecules. These molecules can be decomposed into the elements hydroge ...
... Elements are the simplest forms of matter. They can exist alone or in various combinations. Different elements can chemically combine to form molecules or molecular compounds. For example, water is a compound, consisting of water molecules. These molecules can be decomposed into the elements hydroge ...
Assignment # 6 Atomic Structure Drill
... The atomic mass values listed on the periodic table are what are known as “weighted” averages of the naturally occurring masses of the isotopes. You are somewhat familiar with the notion of a weighted average. Many of your course grades are determined this way. If your teacher says to you “tests are ...
... The atomic mass values listed on the periodic table are what are known as “weighted” averages of the naturally occurring masses of the isotopes. You are somewhat familiar with the notion of a weighted average. Many of your course grades are determined this way. If your teacher says to you “tests are ...
Chapter 3
... When two protons are extremely close to each other, there is a strong attraction between them. A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. ...
... When two protons are extremely close to each other, there is a strong attraction between them. A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. ...
Document
... • Add together the reduction halfreaction with the oxidation halfreaction to get the complete redox reaction. ...
... • Add together the reduction halfreaction with the oxidation halfreaction to get the complete redox reaction. ...
Unit 7 Notes - Mahtomedi High School
... • In 1912 Bohr joined Rutherford. He realized that Rutherford's model wasn't quite right. The orbiting electrons should give off energy and eventually spiral down into the nucleus, making the atom collapse. Or the electrons could be knocked out of position if a charged particle passed by. • Boh ...
... • In 1912 Bohr joined Rutherford. He realized that Rutherford's model wasn't quite right. The orbiting electrons should give off energy and eventually spiral down into the nucleus, making the atom collapse. Or the electrons could be knocked out of position if a charged particle passed by. • Boh ...
CHEM 1405 Practice Exam #2 (2015)
... 10) How many valence electrons does the representative element with the electron configuration 1s22s22p63s23p5 possess? A) 5 B) 6 11) The compound Au2Se3 is classified as which of the following? A) binary ionic B) ternary ionic C) 7 D) 2 C) binary molecular D) binary acid 12) Which of the following ...
... 10) How many valence electrons does the representative element with the electron configuration 1s22s22p63s23p5 possess? A) 5 B) 6 11) The compound Au2Se3 is classified as which of the following? A) binary ionic B) ternary ionic C) 7 D) 2 C) binary molecular D) binary acid 12) Which of the following ...