CHEM 1405 Practice Exam #2 (2015)
... 10) How many valence electrons does the representative element with the electron configuration 1s22s22p63s23p5 possess? A) 5 B) 6 11) The compound Au2Se3 is classified as which of the following? A) binary ionic B) ternary ionic C) 7 D) 2 C) binary molecular D) binary acid 12) Which of the following ...
... 10) How many valence electrons does the representative element with the electron configuration 1s22s22p63s23p5 possess? A) 5 B) 6 11) The compound Au2Se3 is classified as which of the following? A) binary ionic B) ternary ionic C) 7 D) 2 C) binary molecular D) binary acid 12) Which of the following ...
Is Kr the symbol for Kryptonite?
... • True or False? The mass number of a nucleus represents the number of protons in the nucleus • Are all atoms of the same element identical? If not, how do they differ? Is this consistent with Dalton’s atomic theory? • True or False? Isotopes of an element have different chemical properties • Tr ...
... • True or False? The mass number of a nucleus represents the number of protons in the nucleus • Are all atoms of the same element identical? If not, how do they differ? Is this consistent with Dalton’s atomic theory? • True or False? Isotopes of an element have different chemical properties • Tr ...
1411_lecture_ch2
... Rutherford. He noticed that when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He t ...
... Rutherford. He noticed that when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He t ...
printer-friendly version of benchmark
... Metalloids are also known as semimetals; having both physical and chemical properties of both metals and nonmetals. Elements of this classification may be shiny or dull, and typically conduct heat and electricity better than nonmetals. Elements in the boron group (group 13) consist of boron as the o ...
... Metalloids are also known as semimetals; having both physical and chemical properties of both metals and nonmetals. Elements of this classification may be shiny or dull, and typically conduct heat and electricity better than nonmetals. Elements in the boron group (group 13) consist of boron as the o ...
... questions had already come to mind in 1898, when J. J. Thomson isolated the electron. That was the first solid proof that atoms are indeed built of much tinier pieces. Thomson speaks of the electron in this recorded passage... Could anything at first sight seem more impractical than a body which is ...
Hein and Arena - faculty at Chemeketa
... Surrounding the atomic nucleus are electrons. The name electron comes from the Greek word for amber, a brownish-yellow fossil resin studied by the early Greeks. They found that when amber was rubbed by a piece of cloth, it attracted such things as bits of straw. This phenomenon, known as the amber ...
... Surrounding the atomic nucleus are electrons. The name electron comes from the Greek word for amber, a brownish-yellow fossil resin studied by the early Greeks. They found that when amber was rubbed by a piece of cloth, it attracted such things as bits of straw. This phenomenon, known as the amber ...
File
... why. Answer the following questions. 1. What does the word “atomic” refer to? 2. What does the pre-fix “sub” mean? 3. What does the word “particle” mean? (Think back to Unit 1) 4. Put them together... what is a “subatomic” particle? 5. There are three types of subatomic particles, what are they? Eac ...
... why. Answer the following questions. 1. What does the word “atomic” refer to? 2. What does the pre-fix “sub” mean? 3. What does the word “particle” mean? (Think back to Unit 1) 4. Put them together... what is a “subatomic” particle? 5. There are three types of subatomic particles, what are they? Eac ...
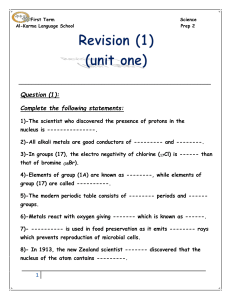
First Term Science Al-Karma Language School Prep 2 Question (1
... 6)-Metals react with oxygen giving ------- which is known as ------. 7)- ---------- is used in food preservation as it emits -------- rays which prevents reproduction of microbial cells. 8)- In 1913, the new Zealand scientist ------- discovered that the nucleus of the atom contains ---------. ...
... 6)-Metals react with oxygen giving ------- which is known as ------. 7)- ---------- is used in food preservation as it emits -------- rays which prevents reproduction of microbial cells. 8)- In 1913, the new Zealand scientist ------- discovered that the nucleus of the atom contains ---------. ...
PSI AP Chemistry Name Unit 4: Chemical Bonding MC Review Part
... same composition. This difference in boiling points may be attributed to a difference in (A) molecular mass (B) density (C) specific heat (D) hydrogen bonding (E) heat of combustion 80. In which of the following molecules is hydrogen bonding likely to be the most significant component of the total i ...
... same composition. This difference in boiling points may be attributed to a difference in (A) molecular mass (B) density (C) specific heat (D) hydrogen bonding (E) heat of combustion 80. In which of the following molecules is hydrogen bonding likely to be the most significant component of the total i ...
Bonding-and-Intermolecular-Forces
... What is hydrogen bonding? When hydrogen bonds to nitrogen, oxygen or fluorine, a larger dipole occurs than in other polar bonds. This is because these atoms are highly electronegative due to their high nuclear charge and small size. When these atoms bond to hydrogen, electrons are withdrawn from th ...
... What is hydrogen bonding? When hydrogen bonds to nitrogen, oxygen or fluorine, a larger dipole occurs than in other polar bonds. This is because these atoms are highly electronegative due to their high nuclear charge and small size. When these atoms bond to hydrogen, electrons are withdrawn from th ...
Electrons - TeacherWeb
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
Atoms, Molecules and Ions Chapter 2
... • Name according to number of carbons, end in –ane. • After 4 carbons names as for other compounds. ...
... • Name according to number of carbons, end in –ane. • After 4 carbons names as for other compounds. ...
Electrons - Chemistry Geek
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
`plum pudding` model - School District of Clayton
... qualities. Fire, water, earth, and air. • The atom receded into the background. ...
... qualities. Fire, water, earth, and air. • The atom receded into the background. ...
Chapter 1 (Matter and Measurement) Objectives
... Know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E=mc2) is small but significant in nuclear reactions. Understand the terms nuclear fission and fusion Understand, that in very general term ...
... Know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E=mc2) is small but significant in nuclear reactions. Understand the terms nuclear fission and fusion Understand, that in very general term ...
Chem101 - Lecture 2 Elements Elements as Pure
... isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one has 12 neutrons and represents 78.99% of all natura ...
... isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one has 12 neutrons and represents 78.99% of all natura ...
ch 4 notes sept 30 oct 1.notebook
... periodic table: a chart that organizes all known elements into a grid of horizontal rows (periods) and vertical columns (groups or families) arranged by increasing atomic number ...
... periodic table: a chart that organizes all known elements into a grid of horizontal rows (periods) and vertical columns (groups or families) arranged by increasing atomic number ...
Class IX Chemistry Chapter 4: Structure of the Atom
... Ernest Rutherford and his co-workers were working in the area of radioactivity. They were studying the effect of alpha () particles on matter. The alpha particles are helium nuclei, which can be obtained by the removal of two electrons from the helium atom. In 1910, Hans Geiger (Rutherford‟s techni ...
... Ernest Rutherford and his co-workers were working in the area of radioactivity. They were studying the effect of alpha () particles on matter. The alpha particles are helium nuclei, which can be obtained by the removal of two electrons from the helium atom. In 1910, Hans Geiger (Rutherford‟s techni ...
Electrons - Irion County ISD
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
PVS103 - unit 6 notes
... We have already seen how electronegativity increases on moving across the periodic table. Can you remember why? As a result electronegativity is a periodic property, so for example, the halogens (Group 7a elements) are all highly electronegative. ...
... We have already seen how electronegativity increases on moving across the periodic table. Can you remember why? As a result electronegativity is a periodic property, so for example, the halogens (Group 7a elements) are all highly electronegative. ...
Document
... electron is known as a. Low state b. Ground state c. Basement state d. Excited state ...
... electron is known as a. Low state b. Ground state c. Basement state d. Excited state ...
13.1 Fundamental Particles and Forces
... a pure element, they find that the light does not include all colors. Instead, they see a few very specific colors, and the colors are different for different elements (Figure 13.11). Hydrogen has a red line, a green line, a blue and a violet line in a characteristic pattern. Helium and lithium have ...
... a pure element, they find that the light does not include all colors. Instead, they see a few very specific colors, and the colors are different for different elements (Figure 13.11). Hydrogen has a red line, a green line, a blue and a violet line in a characteristic pattern. Helium and lithium have ...