Phy. Sci Mid-term review
... Proton Positive charge mass of 1 amu Electron Negative charge mass of 0 amu Neutron No charge mass of 1 amu 16. Draw a picture of the modern day atom. Should include, p.n.e, orbitals , energy levels, and nucleus 17. Describe an isotope and give two examples. Element with different # of N Carbo ...
... Proton Positive charge mass of 1 amu Electron Negative charge mass of 0 amu Neutron No charge mass of 1 amu 16. Draw a picture of the modern day atom. Should include, p.n.e, orbitals , energy levels, and nucleus 17. Describe an isotope and give two examples. Element with different # of N Carbo ...
Periodic Table - Chemistry R: 4(AE)
... noticed that certain similarities occurred at regular intervals. This was considered a “periodic pattern”. • He arranged all the elements in a table according to increasing atomic mass, starting a new row every time the pattern repeated, and the elements with similar properties fell in the same vert ...
... noticed that certain similarities occurred at regular intervals. This was considered a “periodic pattern”. • He arranged all the elements in a table according to increasing atomic mass, starting a new row every time the pattern repeated, and the elements with similar properties fell in the same vert ...
Periodic Table 2015
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
File
... a decimal). Then, add the results together and round off to an appropriate number of significant figures. This is the solution for carbon: ...
... a decimal). Then, add the results together and round off to an appropriate number of significant figures. This is the solution for carbon: ...
Ionization energy
... (attractive force between the electron and the nucleus is inversely proportional to the distance). As a result it becomes easier to remove the electron and therefore the ionization energy decreases with the increase in atomic size. ...
... (attractive force between the electron and the nucleus is inversely proportional to the distance). As a result it becomes easier to remove the electron and therefore the ionization energy decreases with the increase in atomic size. ...
File
... composed of? What is the structure of material objects? Is there a basic unit from which all objects are made? As early as 400 B.C., some Greek philosophers proposed that matter is made of indivisible building blocks known as atomos. (Atomos in Greek means indivisible.) To these early Greeks, matter ...
... composed of? What is the structure of material objects? Is there a basic unit from which all objects are made? As early as 400 B.C., some Greek philosophers proposed that matter is made of indivisible building blocks known as atomos. (Atomos in Greek means indivisible.) To these early Greeks, matter ...
The Periodic Table - Journigan-wiki
... As the principal quantum number (n) increases, the size of the electron cloud increases. That is, the atomic size increases as you go down the table. The reason for this is that you are adding energy levels as you go down the table (1, 2, 3,...). The positive charge of the nucleus increase as you go ...
... As the principal quantum number (n) increases, the size of the electron cloud increases. That is, the atomic size increases as you go down the table. The reason for this is that you are adding energy levels as you go down the table (1, 2, 3,...). The positive charge of the nucleus increase as you go ...
S8P1-study-guide
... You can find the number of neutrons in an atom by using this formula: Neutrons = mass number (protons + neutrons) – atomic number (protons) B. What is a molecule? Molecules are formed by the combination of one or more types of atoms chemically joined together. Unlike atoms, molecules can be subdivid ...
... You can find the number of neutrons in an atom by using this formula: Neutrons = mass number (protons + neutrons) – atomic number (protons) B. What is a molecule? Molecules are formed by the combination of one or more types of atoms chemically joined together. Unlike atoms, molecules can be subdivid ...
Protons are the identity of an atom!
... All of the atom’s electrons are located somewhere in the electron cloud. It is not possible to know exactly where the electrons of an atom are located. It is useful to think of electrons as orbiting the nucleus much as planets orbit the Sun (as shown in the Bohr Model), though electrons do not reall ...
... All of the atom’s electrons are located somewhere in the electron cloud. It is not possible to know exactly where the electrons of an atom are located. It is useful to think of electrons as orbiting the nucleus much as planets orbit the Sun (as shown in the Bohr Model), though electrons do not reall ...
Ch. 6 - The Periodic Table
... Trends in Ionization Energy ◦ What are the trends among the elements for first ionization energy, ionic size, and electronegativity? The energy required to remove an electron from an atom is called ionization energy. The energy required to remove the first electron from an atom is called the fir ...
... Trends in Ionization Energy ◦ What are the trends among the elements for first ionization energy, ionic size, and electronegativity? The energy required to remove an electron from an atom is called ionization energy. The energy required to remove the first electron from an atom is called the fir ...
Chapter 4 Atomic Structure
... This process is called _______________. This __________ of absorption and emission happens very fast over and over again. Atoms can be excited using _________, light, or ...
... This process is called _______________. This __________ of absorption and emission happens very fast over and over again. Atoms can be excited using _________, light, or ...
Chapter 2 Atoms, Molecules and Ions Atomos “uncuttable” Protons +
... 2. Mass of 1 proton ≈ Mass of 1 neutron 3. e- s are MUCH lighter than protons & neutrons 4. Nucleus consists of protons and neutrons only; nuclei contain all of the positive charge and almost all of the mass of the atom 5. Most of an atom’s volume is empty space ...
... 2. Mass of 1 proton ≈ Mass of 1 neutron 3. e- s are MUCH lighter than protons & neutrons 4. Nucleus consists of protons and neutrons only; nuclei contain all of the positive charge and almost all of the mass of the atom 5. Most of an atom’s volume is empty space ...
The Atom
... what is the charge of the resulting ion? 2) How many electrons would be found in the ion O2-? 3) If an ion has 28 protons and 26 electrons, what is its charge? What is its symbol (including charge)? ...
... what is the charge of the resulting ion? 2) How many electrons would be found in the ion O2-? 3) If an ion has 28 protons and 26 electrons, what is its charge? What is its symbol (including charge)? ...
Investigating Atoms and Atomic Theory
... A space in which electrons are likely to be found. Electrons whirl about the nucleus billions of times in one second They are not moving around in random patterns. Location of electrons depends ...
... A space in which electrons are likely to be found. Electrons whirl about the nucleus billions of times in one second They are not moving around in random patterns. Location of electrons depends ...
Study Guide-Chemistry Of Life
... 10. Are the CHNOPS elements stable and unreactive? What are they and why? Why is this important for living things? ...
... 10. Are the CHNOPS elements stable and unreactive? What are they and why? Why is this important for living things? ...
Atomic Theory - World of Teaching
... Which statement is correct concerning the mass of a ball of clay? The mass changes as the altitude of the ball of clay changes. The mass changes as the shape of the ball of clay changes. The mass of the ball of clay is unchanged by altitude or shape. The mass is doubled when the ball of clay is div ...
... Which statement is correct concerning the mass of a ball of clay? The mass changes as the altitude of the ball of clay changes. The mass changes as the shape of the ball of clay changes. The mass of the ball of clay is unchanged by altitude or shape. The mass is doubled when the ball of clay is div ...
Matter
... idea that matter is formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means “uncuttable,” for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The Greek idea of atoms had to wait about 2,000 years before it ...
... idea that matter is formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means “uncuttable,” for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The Greek idea of atoms had to wait about 2,000 years before it ...
Atomic Theory Lecture Notes
... and chemical properties. 3. Atoms of different elements have different physical and chemical properties. 4. Atoms of different elements combine in simple whole number ratios to form chemical compounds. 5. In chemical reactions, atoms cannot be subdivided, created, or destroyed. They are combined, se ...
... and chemical properties. 3. Atoms of different elements have different physical and chemical properties. 4. Atoms of different elements combine in simple whole number ratios to form chemical compounds. 5. In chemical reactions, atoms cannot be subdivided, created, or destroyed. They are combined, se ...
Nature of Atoms Atomic Structure
... – Any substance that cannot be broken down to any other substance by ordinary chemical means ...
... – Any substance that cannot be broken down to any other substance by ordinary chemical means ...
Chapter 2 Atoms, Ions, and the Periodic Table
... The first draft of the periodic table was developed between 1879 and 1871, and published by Dmitri Mendeleev. Note that this was before the subatomic particles were discovered, so it was not based on atomic number. The 63 known elements were arranged in order of increasing relative atomic mass, ...
... The first draft of the periodic table was developed between 1879 and 1871, and published by Dmitri Mendeleev. Note that this was before the subatomic particles were discovered, so it was not based on atomic number. The 63 known elements were arranged in order of increasing relative atomic mass, ...
HISTORICAL OVERVIEW OF THE ATOM
... The number of protons in an atom is equal to the number of electrons, to balance the charge. ...
... The number of protons in an atom is equal to the number of electrons, to balance the charge. ...
Isotope Practice Worksheet
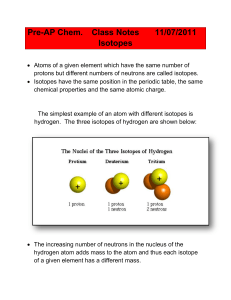
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...