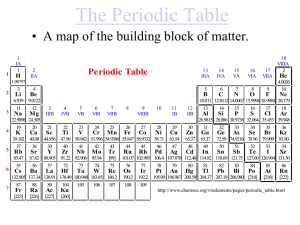
Medical Chemistry Lecture By : Asst. LectTariq Al Mgheer of
... hydrogen is 1; of helium 2; of lithium 3. The atomic number of each element is given in the periodic table (it is the number placed directly below the symbol of the element periodic table). All the atoms of an element have the same atomic number. The importance of this statement became clear with th ...
... hydrogen is 1; of helium 2; of lithium 3. The atomic number of each element is given in the periodic table (it is the number placed directly below the symbol of the element periodic table). All the atoms of an element have the same atomic number. The importance of this statement became clear with th ...
Early Atomic Theory - Cinnaminson School
... His experiments also revealed that the combined masses of mercury (200.59) and oxygen (16.00) were exactly equal to the mass of calx of mercury (216.59). That is, there was no change in mass upon formation or decomposition of the calx. Lavoisier hypothesized that this should be true of all chemical ...
... His experiments also revealed that the combined masses of mercury (200.59) and oxygen (16.00) were exactly equal to the mass of calx of mercury (216.59). That is, there was no change in mass upon formation or decomposition of the calx. Lavoisier hypothesized that this should be true of all chemical ...
Periodic_Tendancies
... atoms are in upper right corner of periodic table (fluorine) • That’s why atoms on the right gain electrons; they pull electrons from the metals on the left. ...
... atoms are in upper right corner of periodic table (fluorine) • That’s why atoms on the right gain electrons; they pull electrons from the metals on the left. ...
Atomic Structure Review–Honors
... – more than one element – elements combined in definite proportions • Molecule: – Smallest unit of a compound that still retains the properties of the compound. ...
... – more than one element – elements combined in definite proportions • Molecule: – Smallest unit of a compound that still retains the properties of the compound. ...
Chapter 8
... each atom achieves an octet (duet). • Coordinate covalent bond forms when one atom provides both bonding electrons. • Multiple covalent bond forms when more than one electron pair is shared between two atoms (double bond, bond order 2 [CO2] and triple bond, bond order 3 [N2]). ...
... each atom achieves an octet (duet). • Coordinate covalent bond forms when one atom provides both bonding electrons. • Multiple covalent bond forms when more than one electron pair is shared between two atoms (double bond, bond order 2 [CO2] and triple bond, bond order 3 [N2]). ...
Investigating Atoms and Atomic Theory
... The Wave Model In fact, it is impossible to determine the exact location of an electron. The probable location of an electron is based on how much energy the electron has. According to the modern atomic model, at atom has a small positively charged nucleus surrounded by a large region in which ther ...
... The Wave Model In fact, it is impossible to determine the exact location of an electron. The probable location of an electron is based on how much energy the electron has. According to the modern atomic model, at atom has a small positively charged nucleus surrounded by a large region in which ther ...
Honors Chem: Atomic History-Isotopes
... A patient is administered 20 mg of iodone-131. How much of this isotope remains after in the body after 40 days if the half life of I-131 is 8 days? What was the original mass of a substance if after 7.5 days 12 grams remains? [the half life of this substance is 2.5 days.] Manganese-56 is a beta emi ...
... A patient is administered 20 mg of iodone-131. How much of this isotope remains after in the body after 40 days if the half life of I-131 is 8 days? What was the original mass of a substance if after 7.5 days 12 grams remains? [the half life of this substance is 2.5 days.] Manganese-56 is a beta emi ...
File
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
Chapter 2
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
Chapter 3: Atoms & the Periodic Table
... • The number of protons in an element does not change • the number of neutrons can change. (Isotopes of an atom) –Ex: Carbon atoms always have 6 protons. But they may have 5,6,7,or 8 neutrons. (Isotopes of carbon) • This means that the amu will vary. ...
... • The number of protons in an element does not change • the number of neutrons can change. (Isotopes of an atom) –Ex: Carbon atoms always have 6 protons. But they may have 5,6,7,or 8 neutrons. (Isotopes of carbon) • This means that the amu will vary. ...
Grade 9 Science Unit: Atoms and Elements Topic 4: Periodic Table
... - The rule about capitalization is very important. Ex. The symbol _________ stands for _____________, while _______ represents ________________ (a compound that contains both ___________ and _____________) - The symbol is sometimes an __________________ of the elements English name, other times it a ...
... - The rule about capitalization is very important. Ex. The symbol _________ stands for _____________, while _______ represents ________________ (a compound that contains both ___________ and _____________) - The symbol is sometimes an __________________ of the elements English name, other times it a ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... The Mass Number is an integral number used to indicate the approximate mass of the atom = sum of protons and neutrons in the nucleus. Example: 126C is carbon, atomic number = 6, mass number = 12. (Also called carbon-12). It comprises 6 protons, 6 neutrons, and 6 electrons. ...
... The Mass Number is an integral number used to indicate the approximate mass of the atom = sum of protons and neutrons in the nucleus. Example: 126C is carbon, atomic number = 6, mass number = 12. (Also called carbon-12). It comprises 6 protons, 6 neutrons, and 6 electrons. ...
File
... b. Dashed lines for a property indicate that data is available. Some elements, for example, may not form a compound with hydrogen. 3. Working together, discuss the possibilities for arrangement of the element cards with all members of the group, and look for a logical arrangement of the cards. Consi ...
... b. Dashed lines for a property indicate that data is available. Some elements, for example, may not form a compound with hydrogen. 3. Working together, discuss the possibilities for arrangement of the element cards with all members of the group, and look for a logical arrangement of the cards. Consi ...
Elements and Atoms
... theorize that matter was made of small pieces. Leucippus was the first to use the term atom (atomon), which meant "indivisible" in Greek. We now know that the atom is divisible and is made of even smaller pieces — the puzzling subatomic particles. Because the Greeks had no way to test and verify the ...
... theorize that matter was made of small pieces. Leucippus was the first to use the term atom (atomon), which meant "indivisible" in Greek. We now know that the atom is divisible and is made of even smaller pieces — the puzzling subatomic particles. Because the Greeks had no way to test and verify the ...
atoms
... Most of mass and all of positive charge of an atom are centered in a very small region called nucleus. The remainder of the atom is mostly empty space The magnitude of the positive charge is different for the different atoms and is approximately one-half the atomic weight of the element There a ...
... Most of mass and all of positive charge of an atom are centered in a very small region called nucleus. The remainder of the atom is mostly empty space The magnitude of the positive charge is different for the different atoms and is approximately one-half the atomic weight of the element There a ...
Isotopes - Katella HS
... Same but different? Pre-1982 penny = 3.11 g Post-1982 penny = 2.55 g Same coin…why? Inside different: Pre-1982 all Cu Post-1982 Zn & Cu ...
... Same but different? Pre-1982 penny = 3.11 g Post-1982 penny = 2.55 g Same coin…why? Inside different: Pre-1982 all Cu Post-1982 Zn & Cu ...
atoms
... proportions by mass of the constituent elements. Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two elements Exp: determine the percent by mass of h ...
... proportions by mass of the constituent elements. Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two elements Exp: determine the percent by mass of h ...
Chapter 4 Atomic Structure
... abbreviated as “AMN”. • As it’s name implies, it represents the MASS OF ONE ATOM. The unit for AMN is a derived unit known as the ATOMIC MASS UNIT, or simply “amu” or more simply “u”. • The AMN is calculated by adding the total number of PROTONS and NEUTRONS in the atom together. • On the Periodic T ...
... abbreviated as “AMN”. • As it’s name implies, it represents the MASS OF ONE ATOM. The unit for AMN is a derived unit known as the ATOMIC MASS UNIT, or simply “amu” or more simply “u”. • The AMN is calculated by adding the total number of PROTONS and NEUTRONS in the atom together. • On the Periodic T ...
File
... 1. If an atom has an atomic number of 8, how many protons does the atom have? _______ protons. a. Which is also equal to the number of (circle all that apply) Protons Neutrons Electrons 2. What are the three sub-atomic particles and why are they identified as sub-atomic? ____________________________ ...
... 1. If an atom has an atomic number of 8, how many protons does the atom have? _______ protons. a. Which is also equal to the number of (circle all that apply) Protons Neutrons Electrons 2. What are the three sub-atomic particles and why are they identified as sub-atomic? ____________________________ ...
File - Mr. Dang`s Science Classroom Website
... Atomic Theory: Most of the matter of the atom is found in a very small part of the atom. This is called the nucleus of the atom. It is very tiny and extremely dense. Result: Some of the positively charged particles were deflected or even ...
... Atomic Theory: Most of the matter of the atom is found in a very small part of the atom. This is called the nucleus of the atom. It is very tiny and extremely dense. Result: Some of the positively charged particles were deflected or even ...
Name______________________ Making - Science
... one neutron in its nucleus is called Deuterium. Deuterium is not radioactive. Water made from deuterium is called heavy water because the extra neutron makes it heavier. It is used in nuclear reactors. The third isotope of hydrogen is known as Tritium. It has one proton and two neutrons in its nucle ...
... one neutron in its nucleus is called Deuterium. Deuterium is not radioactive. Water made from deuterium is called heavy water because the extra neutron makes it heavier. It is used in nuclear reactors. The third isotope of hydrogen is known as Tritium. It has one proton and two neutrons in its nucle ...
File
... 31. As an electron in a hydrogen atom moves from the second principal energy level to the first principal energy level, the energy of the atom A) decreases B) increases C) remains the same 32. An atom of oxygen is in an excited state. When an electron in this atom moves from the third shell to the s ...
... 31. As an electron in a hydrogen atom moves from the second principal energy level to the first principal energy level, the energy of the atom A) decreases B) increases C) remains the same 32. An atom of oxygen is in an excited state. When an electron in this atom moves from the third shell to the s ...