File
... model is based on the principles of wave mechanics. According to the theory of wave mechanics, electrons do not orbit the atom like planets around the sun. It is impossible to determine the EXACT location of an electron. ...
... model is based on the principles of wave mechanics. According to the theory of wave mechanics, electrons do not orbit the atom like planets around the sun. It is impossible to determine the EXACT location of an electron. ...
Chapter 29: Atomic Structure What will we learn in this chapter
... Electron spin In the 1920s it was noticed that energy levels would split even when no external field was present. This suggests that the electron has an intrinsic angular momentum, in addition to the angular momentum associated with the orbital motion. Picture electrons as charged spinning spheres. ...
... Electron spin In the 1920s it was noticed that energy levels would split even when no external field was present. This suggests that the electron has an intrinsic angular momentum, in addition to the angular momentum associated with the orbital motion. Picture electrons as charged spinning spheres. ...
chapter5 - MrFoti.com
... Counting the Pieces Atomic Number = number of protons in the nucleus # of protons determines kind of atom (since all protons are alike!) the same as the number of electrons in the neutral atom. Mass Number = the number of protons + neutrons in a particular isotope of that element. These acc ...
... Counting the Pieces Atomic Number = number of protons in the nucleus # of protons determines kind of atom (since all protons are alike!) the same as the number of electrons in the neutral atom. Mass Number = the number of protons + neutrons in a particular isotope of that element. These acc ...
electrons - Clay County OutReach
... the 1700’s nearly all chemists had accepted the modern definition of an element as a particle that is indivisible It was also understood at that time that elements combine to form compounds that are different in their properties than the elements that composed them However, ...
... the 1700’s nearly all chemists had accepted the modern definition of an element as a particle that is indivisible It was also understood at that time that elements combine to form compounds that are different in their properties than the elements that composed them However, ...
Atomic structure and radioactive decay
... Notice that the mass number is different. How many neutrons does each isotope have? ...
... Notice that the mass number is different. How many neutrons does each isotope have? ...
Atoms: The Building Blocks of Matter
... 1. Explain the difference between the mass number and the atomic number of a nuclide. Mass number is the total number of protons and neutrons in the nucleus of an isotope. Atomic number is the total number of protons in the nucleus of each atom of an element. ...
... 1. Explain the difference between the mass number and the atomic number of a nuclide. Mass number is the total number of protons and neutrons in the nucleus of an isotope. Atomic number is the total number of protons in the nucleus of each atom of an element. ...
Atomic Structure PPT
... of 78 what is the –number of protons –number of neutrons –number of electrons –Complete symbol ...
... of 78 what is the –number of protons –number of neutrons –number of electrons –Complete symbol ...
Click here to Ch 3.2_ Atoms_Structure
... “The universe is composed of two elements: the atoms and the void in which they exist and move.” Everything is composed of "atoms", which are physically, but not geometrically, indivisible; that between atoms, there lies empty space; that atoms are indestructible, and have always been and always wil ...
... “The universe is composed of two elements: the atoms and the void in which they exist and move.” Everything is composed of "atoms", which are physically, but not geometrically, indivisible; that between atoms, there lies empty space; that atoms are indestructible, and have always been and always wil ...
Ch. 4 Notes – THE STRUCTURE OF THE ATOM NOTE
... Radioactive decay (overlap with Chapter 24) A. Nuclear reactions 1) nuclear reactions—chemical reactions converting matter to energy 2) violate the Conservation Laws 3) involves transmutation—the changing of one element into another element B. Radioisotopes 1) radioisotopes (radioactive isotopes or ...
... Radioactive decay (overlap with Chapter 24) A. Nuclear reactions 1) nuclear reactions—chemical reactions converting matter to energy 2) violate the Conservation Laws 3) involves transmutation—the changing of one element into another element B. Radioisotopes 1) radioisotopes (radioactive isotopes or ...
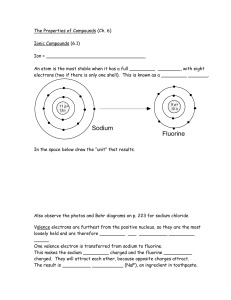
Understanding the Properties of Elements – Chapter 5
... An atom is the most stable when it has a full _________ ________, with eight electrons (two if there is only one shell). This is known as a _________ _______. ...
... An atom is the most stable when it has a full _________ ________, with eight electrons (two if there is only one shell). This is known as a _________ _______. ...
ElectronConfigurationSE
... 9. Infer: Select the PERIODIC TABLE tab. Earlier you saw that the transition metals represent the filling of the d subshells. Now locate the purple lanthanides and actinides on the bottom rows of the periodic table. A. How many elements are in the in the lanthanides series? _____________________ B. ...
... 9. Infer: Select the PERIODIC TABLE tab. Earlier you saw that the transition metals represent the filling of the d subshells. Now locate the purple lanthanides and actinides on the bottom rows of the periodic table. A. How many elements are in the in the lanthanides series? _____________________ B. ...
Electron config atomic structure
... of 78 what is the –number of protons –number of neutrons –number of electrons –Complete symbol ...
... of 78 what is the –number of protons –number of neutrons –number of electrons –Complete symbol ...
Investigating Atoms and Atomic Theory
... sheet were mostly open space. Atoms were not a pudding filled with a positively charged ...
... sheet were mostly open space. Atoms were not a pudding filled with a positively charged ...
Study guide for periodic table trends. A. By referring to electron
... 17. What increases in equal steps of one from left to right in the periodic table for the elements lithium to neon? a. The number of occupied electron energy levels b. The number of neutrons in the most common isotope c. The number of electrons in the atom d. The atomic mass ...
... 17. What increases in equal steps of one from left to right in the periodic table for the elements lithium to neon? a. The number of occupied electron energy levels b. The number of neutrons in the most common isotope c. The number of electrons in the atom d. The atomic mass ...
Chapter 2 Practice Questions
... A) All samples of chlorine contain 35Cl and 37Cl in the same (definite) ratio. B) The mass of oxygen that is combined with a fixed mass of nitrogen in each of the binary nitrogen oxides can be expressed as a ratio of small whole numbers. C) The atomic masses of all of the elements in the periodic ta ...
... A) All samples of chlorine contain 35Cl and 37Cl in the same (definite) ratio. B) The mass of oxygen that is combined with a fixed mass of nitrogen in each of the binary nitrogen oxides can be expressed as a ratio of small whole numbers. C) The atomic masses of all of the elements in the periodic ta ...
Atomic Structure
... Define the terms mass number (Z) and isotopes of an element. Deduce the symbol for an isotope given it’s mass number and atomic number. Calculate the number of protons, neutrons and electrons in atoms and ions from the mass number, atomic number and charge. ...
... Define the terms mass number (Z) and isotopes of an element. Deduce the symbol for an isotope given it’s mass number and atomic number. Calculate the number of protons, neutrons and electrons in atoms and ions from the mass number, atomic number and charge. ...
Atomic Radii
... What is the definition of the term. Where are the atoms located on the periodic table (and valence electrons). How this affects the stated trend. Why it affects the stated trend and relate back to question. Sodium atom and sodium ions have the same number of protons but the sodium ion has one less e ...
... What is the definition of the term. Where are the atoms located on the periodic table (and valence electrons). How this affects the stated trend. Why it affects the stated trend and relate back to question. Sodium atom and sodium ions have the same number of protons but the sodium ion has one less e ...
Chemistry Cram Sheet
... Used to tell how “off” you are from the value you should have gotten. Used mostly in lab. Ex: The specific heat capacity of iron is 0.45 J/gC. A student uses a calorimeter to experimentally determine the specific heat of iron to be 0.60 J/gC. What is the student’s percent error? ...
... Used to tell how “off” you are from the value you should have gotten. Used mostly in lab. Ex: The specific heat capacity of iron is 0.45 J/gC. A student uses a calorimeter to experimentally determine the specific heat of iron to be 0.60 J/gC. What is the student’s percent error? ...
The Chemistry of Life
... 3) How can we tell that this atom may be an isotope? a) there are more neutrons than protons b) there are more protons than neutrons c) there are enough neutrons to balance the charge of the protons d) this atom has an overall negative charge ...
... 3) How can we tell that this atom may be an isotope? a) there are more neutrons than protons b) there are more protons than neutrons c) there are enough neutrons to balance the charge of the protons d) this atom has an overall negative charge ...
Name: ___________ Class: _____ Date: _______________ FALL
... c. sugar is dissolved in a solution. d. an unexpected color change occurs. ____ 11. Which sign does NOT indicate that a chemical change has occurred? a. color change. c. energy absorbed or released. b. dissolving in a solution. d. gas produced. ____ 12. A physical change occurs when a. milk sours. c ...
... c. sugar is dissolved in a solution. d. an unexpected color change occurs. ____ 11. Which sign does NOT indicate that a chemical change has occurred? a. color change. c. energy absorbed or released. b. dissolving in a solution. d. gas produced. ____ 12. A physical change occurs when a. milk sours. c ...
Chapter 4 - H - Regional School District 17
... No matter what metal was used, the particles had a mass 1/2000 of a Hydrogen atom. The beam was always attracted to the positive side Hypothesis- these particles came from the atoms. ...
... No matter what metal was used, the particles had a mass 1/2000 of a Hydrogen atom. The beam was always attracted to the positive side Hypothesis- these particles came from the atoms. ...
Procedure
... Graph 3 - For elements 3-20, make a graph of the energy required to remove the easiest electron (first ionization energy) as a function of atomic number. Plot atomic number on the X axis and energy required on the Y axis. After creating the graph, use a colored pen or pencil to draw a vertical line ...
... Graph 3 - For elements 3-20, make a graph of the energy required to remove the easiest electron (first ionization energy) as a function of atomic number. Plot atomic number on the X axis and energy required on the Y axis. After creating the graph, use a colored pen or pencil to draw a vertical line ...