chapt 1 - Cantt Academy, Tahli Mohri Chowk, Rawalpindi
... Nuclear Chemistry:The study of the effect of neutrons on vinegar is called nuclear chemistry. ...
... Nuclear Chemistry:The study of the effect of neutrons on vinegar is called nuclear chemistry. ...
Atomic Structure
... but it doesn’t exists as an isotope with 2 neutrons or as an isotope with 5 neutrons. This whole discussion of isotopes brings us back to Dalton’s atomic theory. According to Dalton, atoms of a given element are identical. But if atoms of a given element can have different numbers of neutrons, then ...
... but it doesn’t exists as an isotope with 2 neutrons or as an isotope with 5 neutrons. This whole discussion of isotopes brings us back to Dalton’s atomic theory. According to Dalton, atoms of a given element are identical. But if atoms of a given element can have different numbers of neutrons, then ...
Booklet Chapter 3
... Weighted average mass A mass calculated by multiplying the decimal fraction of each component in a sample by its mass and adding the results of each multiplication together. Atomic mass unit 1/12 the mass of a carbon-12 atom. It is sometimes called a unified mass unit. Its accepted abbreviation is u, ...
... Weighted average mass A mass calculated by multiplying the decimal fraction of each component in a sample by its mass and adding the results of each multiplication together. Atomic mass unit 1/12 the mass of a carbon-12 atom. It is sometimes called a unified mass unit. Its accepted abbreviation is u, ...
atoms - WordPress.com
... All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed around the nucleus, and occupy most of the volume His model was called ...
... All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed around the nucleus, and occupy most of the volume His model was called ...
C5-Early-Atomic-Theory-and-Structure-Comp
... 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make th ...
... 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make th ...
atomic mass
... Some mass is lost in the energy binding nucleons together (E = m c2) The periodic table shows the average atomic masses, in amu. These masses are the weighted averages of the masses of all of the naturally occurring isotopes . © 2014 W. W. Norton Co., Inc. ...
... Some mass is lost in the energy binding nucleons together (E = m c2) The periodic table shows the average atomic masses, in amu. These masses are the weighted averages of the masses of all of the naturally occurring isotopes . © 2014 W. W. Norton Co., Inc. ...
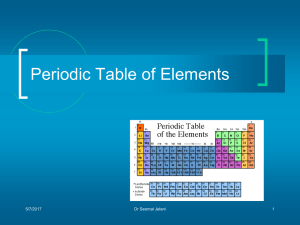
A “periodic table” is an arrangement of elements in
... He grouped elements according to their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
... He grouped elements according to their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
Chapter 2 Atoms, Molecules, and Ions
... (a) The superscript 197 is the mass number (protons + neutrons). According to the list of elements given on the front inside cover, gold has atomic number 79. Consequently, an atom of 197Au has 79 protons, 79 electrons, and 197 – 79 = 118 neutrons. (b) The atomic number of strontium is 38. Thus, all ...
... (a) The superscript 197 is the mass number (protons + neutrons). According to the list of elements given on the front inside cover, gold has atomic number 79. Consequently, an atom of 197Au has 79 protons, 79 electrons, and 197 – 79 = 118 neutrons. (b) The atomic number of strontium is 38. Thus, all ...
Incorrect…try again
... • 8 is the number of neutrons. This does not indicate element name. You need to look at the protons to determine the element name. • Mass # is found by adding protons and neutrons • 12 is only the number of neutrons. You must add the protons to this number. ...
... • 8 is the number of neutrons. This does not indicate element name. You need to look at the protons to determine the element name. • Mass # is found by adding protons and neutrons • 12 is only the number of neutrons. You must add the protons to this number. ...
9.1 REDOX Introduction to Oxidation and Reduction
... 9.1.1 Define oxidation and reduction (electron loss and gain) 9.1.2 Deduce the oxidation number of an element in a compound. 9.1.3 State the names of compounds using oxidation numbers. 9.1.4 Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers, ...
... 9.1.1 Define oxidation and reduction (electron loss and gain) 9.1.2 Deduce the oxidation number of an element in a compound. 9.1.3 State the names of compounds using oxidation numbers. 9.1.4 Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers, ...
atoms - Moodle
... than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not have the same relative number Atoms, M ...
... than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not have the same relative number Atoms, M ...
Hewitt/Lyons/Suchocki/Yeh, Conceptual Integrated Science
... – lightest and most abundant is hydrogen • to date, 115 are known – 90 occur in nature – others produced in laboratory are unstable Words atom and element can be used interchangeably Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley ...
... – lightest and most abundant is hydrogen • to date, 115 are known – 90 occur in nature – others produced in laboratory are unstable Words atom and element can be used interchangeably Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley ...
CP Chemistry - Final Exam Review KEY
... A gas takes up a volume of 17.3 liters, has a pressure of 2.30 atm, and a temperature of 299 K. If I raise the temperature to 350. K and lower the pressure to 1.5 atm, what is the new volume of the gas? Which law is this? ...
... A gas takes up a volume of 17.3 liters, has a pressure of 2.30 atm, and a temperature of 299 K. If I raise the temperature to 350. K and lower the pressure to 1.5 atm, what is the new volume of the gas? Which law is this? ...
Nucleon number
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
atoms
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
file - Mindset Learn
... 10.1 Given the electron configuration, determine the charge when one of these atoms gains or loses electrons to form an ion: Nitrogen: 1s22s22p3 10.2 Given the electron configuration, determine the charge when one of these atoms gains or loses electrons to form an ion: Oxygen: 1s22s22p4 10.3 Given t ...
... 10.1 Given the electron configuration, determine the charge when one of these atoms gains or loses electrons to form an ion: Nitrogen: 1s22s22p3 10.2 Given the electron configuration, determine the charge when one of these atoms gains or loses electrons to form an ion: Oxygen: 1s22s22p4 10.3 Given t ...
ch 3 classification of elements and periodic properties
... No. of periods: 7 No. of Groups: 18 No. of periods represents the highest principal quantum number (n) of the elements present in it. First Period: n=1…..(1s) two elements (h & He) Second Period n=2….(2s & 2p) eight elements (Li to Ne) Third Period n=3 …(3s & 3p) eight elements (Na to Ar) Fourth Per ...
... No. of periods: 7 No. of Groups: 18 No. of periods represents the highest principal quantum number (n) of the elements present in it. First Period: n=1…..(1s) two elements (h & He) Second Period n=2….(2s & 2p) eight elements (Li to Ne) Third Period n=3 …(3s & 3p) eight elements (Na to Ar) Fourth Per ...