Part V Elements And Atomic Weights
... To put it simply, elements are the basic building blocks of the chemical and physical world, as we know it. While many of us remember this basic concept from high school chemistry class, details such as the name, abbreviation, and atomic weight2 of each element are probably a bit fuzzy. This is unde ...
... To put it simply, elements are the basic building blocks of the chemical and physical world, as we know it. While many of us remember this basic concept from high school chemistry class, details such as the name, abbreviation, and atomic weight2 of each element are probably a bit fuzzy. This is unde ...
Name - Films On Demand
... • Students should organize materials and other resources, plan their work, make good use of group collaboration where appropriate, choose suitable tools and techniques, and work with appropriate measurement methods to ensure adequate accuracy. ...
... • Students should organize materials and other resources, plan their work, make good use of group collaboration where appropriate, choose suitable tools and techniques, and work with appropriate measurement methods to ensure adequate accuracy. ...
Unit 4: The Nucleus
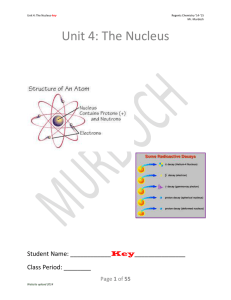
... approximate mass of either a proton or neutron. 3. Atomic Number: The number that identifies an element, equal to an atom’s number of protons. 4. Deflect: Change in direction due to an outside force. 5. Emit: To give off something. 6. Half-life: The time it takes for half the mass of a radioactive i ...
... approximate mass of either a proton or neutron. 3. Atomic Number: The number that identifies an element, equal to an atom’s number of protons. 4. Deflect: Change in direction due to an outside force. 5. Emit: To give off something. 6. Half-life: The time it takes for half the mass of a radioactive i ...
Teacher`s Guide Middle School
... • Students should organize materials and other resources, plan their work, make good use of group collaboration where appropriate, choose suitable tools and techniques, and work with appropriate measurement methods to ensure adequate accuracy. ...
... • Students should organize materials and other resources, plan their work, make good use of group collaboration where appropriate, choose suitable tools and techniques, and work with appropriate measurement methods to ensure adequate accuracy. ...
Book: The Structure of Atoms
... he Dalton theory of the atom and related ideas were the basis for our study of composition stoichiometry (Chapter 2) and reaction stoichiometry (Chapter 3), but that level of atomic theory leaves many questions unanswered. Why do atoms combine to form compounds? Why do they combine only in simple nu ...
... he Dalton theory of the atom and related ideas were the basis for our study of composition stoichiometry (Chapter 2) and reaction stoichiometry (Chapter 3), but that level of atomic theory leaves many questions unanswered. Why do atoms combine to form compounds? Why do they combine only in simple nu ...
lesson plan - cloudfront.net
... from a larger number of atoms. Distribute the same size squares of aluminum foil to each pair of students along with scissors. Have students cut square in half. Then take one half piece and cut it in half and so on until students are unable to make another cut. Students should record the number ...
... from a larger number of atoms. Distribute the same size squares of aluminum foil to each pair of students along with scissors. Have students cut square in half. Then take one half piece and cut it in half and so on until students are unable to make another cut. Students should record the number ...
Chemistry: A Molecular Approach
... in its nucleus • the number of protons in the nucleus of an atom is called the atomic number the elements are arranged on the Periodic Table in order of their atomic numbers ...
... in its nucleus • the number of protons in the nucleus of an atom is called the atomic number the elements are arranged on the Periodic Table in order of their atomic numbers ...
Ionic Bonding - KMChemistryMatters
... 1. Add the valence electrons. 2. Write symbols for the atoms and show which atoms are connected to which. 3. Complete the octet for the central atom then complete the octets of the other atoms. 4. Place leftover electrons on the central atom. 5. If there are not enough electrons to give the central ...
... 1. Add the valence electrons. 2. Write symbols for the atoms and show which atoms are connected to which. 3. Complete the octet for the central atom then complete the octets of the other atoms. 4. Place leftover electrons on the central atom. 5. If there are not enough electrons to give the central ...
1 Unit 2: Atomic Theory Unit Notes Name: Period: ______
... they make up. The following reading passage will help you to better understand the structure of the atom. Understanding Atomic Structure... The word nucleus of the atom is just like the nucleus of the cell that you learned about in Living Environment. One of the properties of the nucleus is that it ...
... they make up. The following reading passage will help you to better understand the structure of the atom. Understanding Atomic Structure... The word nucleus of the atom is just like the nucleus of the cell that you learned about in Living Environment. One of the properties of the nucleus is that it ...
Atoms, Molecules, and Ions
... d. The correct name of this compound in water is hydrobromic acid. e. This is an ionic compound in which the metal cation (Li+) has only one charge. The correct name is lithium carbonate. f. This is an ionic compound in which the metal cation (K) has only one charge. The correct name is potassium d ...
... d. The correct name of this compound in water is hydrobromic acid. e. This is an ionic compound in which the metal cation (Li+) has only one charge. The correct name is lithium carbonate. f. This is an ionic compound in which the metal cation (K) has only one charge. The correct name is potassium d ...
Chapter 2: The Structure of the Atom and the Periodic
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
FREE Sample Here
... 26. What is FALSE about the three orbitals in the 2p sublevel? A) The orbitals have the same dumbbell-like shape. B) The orbitals have similar, but different energies. C) The orbitals are the same distance away from the nucleus. D) The orbitals are each oriented in a different direction. E) Each orb ...
... 26. What is FALSE about the three orbitals in the 2p sublevel? A) The orbitals have the same dumbbell-like shape. B) The orbitals have similar, but different energies. C) The orbitals are the same distance away from the nucleus. D) The orbitals are each oriented in a different direction. E) Each orb ...
ExamView - Periodic Trends Study Guide.tst
... ____ 30. What is the element with the highest electronegativity value? a. cesium b. helium c. calcium d. fluorine ____ 31. Which of the following elements has the smallest ionic radius? a. Li b. K c. O d. S ____ 32. What is the energy required to remove an electron from an atom in the gaseous state ...
... ____ 30. What is the element with the highest electronegativity value? a. cesium b. helium c. calcium d. fluorine ____ 31. Which of the following elements has the smallest ionic radius? a. Li b. K c. O d. S ____ 32. What is the energy required to remove an electron from an atom in the gaseous state ...
Ch 2 Atoms and Elements Student
... How to explain these results Rutherford reasoned that most of the mass of the atom must be concentrated in a relatively ...
... How to explain these results Rutherford reasoned that most of the mass of the atom must be concentrated in a relatively ...
FREE Sample Here
... 26. What is FALSE about the three orbitals in the 2p sublevel? A) The orbitals have the same dumbbell-like shape. B) The orbitals have similar, but different energies. C) The orbitals are the same distance away from the nucleus. D) The orbitals are each oriented in a different direction. E) Each orb ...
... 26. What is FALSE about the three orbitals in the 2p sublevel? A) The orbitals have the same dumbbell-like shape. B) The orbitals have similar, but different energies. C) The orbitals are the same distance away from the nucleus. D) The orbitals are each oriented in a different direction. E) Each orb ...
Chemistry 139
... A negatively charged piece of dust and a positively charged piece of dust A positively charged sodium ion and a positively charged potassium ion A negatively charged chloride ion and a negatively charged bromide ion ...
... A negatively charged piece of dust and a positively charged piece of dust A positively charged sodium ion and a positively charged potassium ion A negatively charged chloride ion and a negatively charged bromide ion ...
Atomic Nucleus and Isotopes
... will be able to discuss their observations, which will help them gain confidence in their results when presenting them to class. This communication is indicative of an actual scientific community in which scientists work together to formulate conclusions. Concepts: The concept of atomic structure an ...
... will be able to discuss their observations, which will help them gain confidence in their results when presenting them to class. This communication is indicative of an actual scientific community in which scientists work together to formulate conclusions. Concepts: The concept of atomic structure an ...
Lecture 1 Atomic Structure
... Since William Crookes, a British physicist, was the first of several scientists to construct discharge tubes, these are also known as Crookes tubes. Working: At ordinary pressure, electricity does not pass through the tube since gases are nonconductor of electricity. As the gas or air from the tube ...
... Since William Crookes, a British physicist, was the first of several scientists to construct discharge tubes, these are also known as Crookes tubes. Working: At ordinary pressure, electricity does not pass through the tube since gases are nonconductor of electricity. As the gas or air from the tube ...
Lab 1
... Data for Lab 1: Dimensional Analysis.................................................................................................... 11 ADDITION AND SUBTRACTION...................................................................................................... 12 MULTIPLICATION & DIVISION OF S ...
... Data for Lab 1: Dimensional Analysis.................................................................................................... 11 ADDITION AND SUBTRACTION...................................................................................................... 12 MULTIPLICATION & DIVISION OF S ...
General and Organic Chemistry Review Primer
... chemist John Dalton, is defined as one twelfth of the mass of an atom of carbon-12. Because the isotopes of an element do not occur with equal frequency, the average atomic mass unit (the weighted average of the atomic masses of the naturally occurring isotopes) is used. For example, hydrogen has th ...
... chemist John Dalton, is defined as one twelfth of the mass of an atom of carbon-12. Because the isotopes of an element do not occur with equal frequency, the average atomic mass unit (the weighted average of the atomic masses of the naturally occurring isotopes) is used. For example, hydrogen has th ...
Chapter 4 What Are Atoms?
... • Explain why some atoms gain or lose electrons to form ions. • Determine how many protons, neutrons, and electrons an atom has, given its symbol, atomic number, and mass number. • Describe how the abundance of isotopes affects an element’s average atomic mass. Chapter menu ...
... • Explain why some atoms gain or lose electrons to form ions. • Determine how many protons, neutrons, and electrons an atom has, given its symbol, atomic number, and mass number. • Describe how the abundance of isotopes affects an element’s average atomic mass. Chapter menu ...
2 - TEST BANK 360
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
Atomic Mass
... The atomic number tells you the number of protons in one an atom of an element. It also tells you the number of electrons in a neutral atom of that element. The atomic number gives the identity of an element as well as its location on the periodic table. No two different elements will have the same ...
... The atomic number tells you the number of protons in one an atom of an element. It also tells you the number of electrons in a neutral atom of that element. The atomic number gives the identity of an element as well as its location on the periodic table. No two different elements will have the same ...