FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A compound that forms between a non-metal and a non-metal is a molecular compound. 35. Stoichiometric coefficients found in a balanced equation can be used to derive mo ...
... 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A compound that forms between a non-metal and a non-metal is a molecular compound. 35. Stoichiometric coefficients found in a balanced equation can be used to derive mo ...
Science SOL CH
... o What factors influence the stability of a nuclide? o What are the different types of nuclear decay? o What happens when an atom undergoes nuclear decay? o Is there a way to predict what type of decay a particular radioisotope will undergo? o How can I use half-life data to calculate the amount of ...
... o What factors influence the stability of a nuclide? o What are the different types of nuclear decay? o What happens when an atom undergoes nuclear decay? o Is there a way to predict what type of decay a particular radioisotope will undergo? o How can I use half-life data to calculate the amount of ...
C H A P T E R
... In 1869, the Russian chemist Dmitri Mendeleev used Newlands’s observation and other information to produce the first orderly arrangement, or periodic table, of all 63 elements known at the time. Mendeleev wrote the symbol for each element, along with the physical and chemical properties and the rela ...
... In 1869, the Russian chemist Dmitri Mendeleev used Newlands’s observation and other information to produce the first orderly arrangement, or periodic table, of all 63 elements known at the time. Mendeleev wrote the symbol for each element, along with the physical and chemical properties and the rela ...
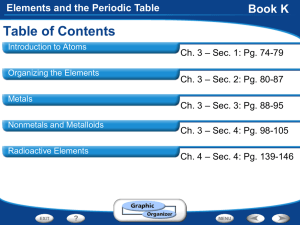
Elements and the Periodic Table
... idea of a fundamental building-block of matter. They reasoned that if you cut an object in half, and kept doing so, you would reach a point at which the object could no longer be cut. This speck of object was called atomos, meaning “uncuttable.” The idea of atomos—atoms—persisted among some thinkers ...
... idea of a fundamental building-block of matter. They reasoned that if you cut an object in half, and kept doing so, you would reach a point at which the object could no longer be cut. This speck of object was called atomos, meaning “uncuttable.” The idea of atomos—atoms—persisted among some thinkers ...
Chapter 3 – Atomic Structure and Properties
... Core electrons shield valence electrons better than do other valence electrons. The nuclear charge experienced by an electron affects the size and energy of its orbitals, so it is an important factor in determining the properties of the valence electrons and orbitals. However, a valence electron is ...
... Core electrons shield valence electrons better than do other valence electrons. The nuclear charge experienced by an electron affects the size and energy of its orbitals, so it is an important factor in determining the properties of the valence electrons and orbitals. However, a valence electron is ...
Atoms
... For example, what smallest possible unit properties. into which a long essay can be divided and still have some meaning? • Made up of: ...
... For example, what smallest possible unit properties. into which a long essay can be divided and still have some meaning? • Made up of: ...
Section 2 Electron Configuration and the Periodic Table Chapter 5
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Condition - Future Website of mrbentley2
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
as PDF - Halbleiter.org
... The periodic table of the chemical elements (periodic table) lists all the chemical elements with increasing proton number (atomic number) and according to their chemical properties, divided into periods as well as main and subgroups. The period represents the number of electron shells, the main gro ...
... The periodic table of the chemical elements (periodic table) lists all the chemical elements with increasing proton number (atomic number) and according to their chemical properties, divided into periods as well as main and subgroups. The period represents the number of electron shells, the main gro ...
Elements and the Periodic Table
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
Scheme of work
... Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name ‘proton’ was given to these particles. ...
... Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name ‘proton’ was given to these particles. ...
ATOMIC THEORY
... Cobalt-60 has a half-life of 5.27 years. How much of a 10.0 g sample will remain after 21.08 years? ...
... Cobalt-60 has a half-life of 5.27 years. How much of a 10.0 g sample will remain after 21.08 years? ...
Dmitri Mendeleev
... Mendeleev noticed that patterns appeared when the elements were arranged in order of increasing atomic mass. As he laid out cards, each element had properties similar to the elements above and below it. Mendeleev's table was not perfect, however. Arranging the elements by increasing atomic mass left ...
... Mendeleev noticed that patterns appeared when the elements were arranged in order of increasing atomic mass. As he laid out cards, each element had properties similar to the elements above and below it. Mendeleev's table was not perfect, however. Arranging the elements by increasing atomic mass left ...
classification of elements and periodicity in properties
... Such work is carried out with competitive spirit only in some laboratories in the world. Scientists, before collecting the reliable data on the new element, at times get tempted to claim for its discovery. For example, both American and Soviet scientists claimed credit for discovering element 104. T ...
... Such work is carried out with competitive spirit only in some laboratories in the world. Scientists, before collecting the reliable data on the new element, at times get tempted to claim for its discovery. For example, both American and Soviet scientists claimed credit for discovering element 104. T ...
Chapter 2 PowerPoint
... Determine (a) the number of moles of C in 25.00 g of carbon, (b) the number of moles of He in 10.50 g of helium, and (c) the number of moles of Na in 15.75 g of sodium. Strategy Molar mass of an element is numerically equal to its average atomic mass. Use the molar mass for each element to convert f ...
... Determine (a) the number of moles of C in 25.00 g of carbon, (b) the number of moles of He in 10.50 g of helium, and (c) the number of moles of Na in 15.75 g of sodium. Strategy Molar mass of an element is numerically equal to its average atomic mass. Use the molar mass for each element to convert f ...
Topic 2.3 The Atom Electron Configuration
... Bohr realised that the electrons could only be at specific energy levels (or states) around the atom. ...
... Bohr realised that the electrons could only be at specific energy levels (or states) around the atom. ...
Chapter 4- Elements and the Periodic Table
... formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means "uncuttable;' for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The ancient Greeks did not prove the existence of atoms because they did not do exp ...
... formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means "uncuttable;' for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The ancient Greeks did not prove the existence of atoms because they did not do exp ...
Chapter 11 Atoms, Energy and Electron Configurations
... Pauli Exclusion Principle • Pauli Exclusion Principle (Wolfgang Pauli 1925) - an atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins • When an orbital contains two electrons (of opposite spin) it is said to be full ...
... Pauli Exclusion Principle • Pauli Exclusion Principle (Wolfgang Pauli 1925) - an atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins • When an orbital contains two electrons (of opposite spin) it is said to be full ...
Now
... ‘d block’ elements are called outer transition elements because they contain at most two electrons in their outer shell. The elements for which f sub shells are filling are called the inner transition elements. Based on electronic configuration, all elements are grouped into four categories. They ar ...
... ‘d block’ elements are called outer transition elements because they contain at most two electrons in their outer shell. The elements for which f sub shells are filling are called the inner transition elements. Based on electronic configuration, all elements are grouped into four categories. They ar ...