2.ATOMS, MOLECULES, AND IONS
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
chapter4
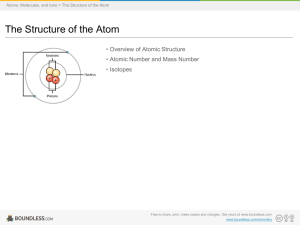
... Explained the nuclear atom. Atom has a dense center of positive charge called the nucleus. Electrons travel around the nucleus in the ...
... Explained the nuclear atom. Atom has a dense center of positive charge called the nucleus. Electrons travel around the nucleus in the ...
elements of chemistry unit
... Sometimes it is useful to assign oxidation numbers to elements found in polar covalent species. By creating Lewis Dot Structures (LDS) diagrams for each element, it is possible to determine their oxidation numbers. Next, combine the LDS diagrams for the elements and determine which electrons are sha ...
... Sometimes it is useful to assign oxidation numbers to elements found in polar covalent species. By creating Lewis Dot Structures (LDS) diagrams for each element, it is possible to determine their oxidation numbers. Next, combine the LDS diagrams for the elements and determine which electrons are sha ...
Section 3 Electron Configurations
... In quantum mechanics, the electrons occupy specific energy levels (as in Bohr's model) but they also exist within specific probability volumes called orbitals with specific orientations in space. The electrons within each orbital has a distinct spin. ...
... In quantum mechanics, the electrons occupy specific energy levels (as in Bohr's model) but they also exist within specific probability volumes called orbitals with specific orientations in space. The electrons within each orbital has a distinct spin. ...
Atomic Structure
... By the late 1700s, chemists had accepted the definition of an element as a substance that cannot be broken down into simpler substances by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical pro ...
... By the late 1700s, chemists had accepted the definition of an element as a substance that cannot be broken down into simpler substances by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical pro ...
Hybridization and St..
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
Lecture Notes
... Dmitri Mendeleev and Julius Lothar Meyer independently proposed ideas or relationships regarding the periodicity of __________ and increasing atomic ________ or atomic _________. Modern Periodic Law states that when elements are arranged in order of increasing atomic number, elements with similar ch ...
... Dmitri Mendeleev and Julius Lothar Meyer independently proposed ideas or relationships regarding the periodicity of __________ and increasing atomic ________ or atomic _________. Modern Periodic Law states that when elements are arranged in order of increasing atomic number, elements with similar ch ...
Chem101 - Lecture 2 Elements Elements
... written in Exercise 2.44 and the factor-unit method to determine the following (The factorunit method is discussed in Study Skills 2.1.): a. The mass in grams of one bromine atom b. The number ot grams of carbon in 2.75 mol of carbon c. The total mass in grams of one-half Avogadro’s number of silver ...
... written in Exercise 2.44 and the factor-unit method to determine the following (The factorunit method is discussed in Study Skills 2.1.): a. The mass in grams of one bromine atom b. The number ot grams of carbon in 2.75 mol of carbon c. The total mass in grams of one-half Avogadro’s number of silver ...
Chapter 6: The Periodic Table and Periodic Law
... Meyer, Mendeleev, and Moseley In 1869, German chemist Lothar Meyer (1830–1895) and Russian chemist Dmitri Mendeleev (1834 –1907) each demonstrated a connection between atomic mass and elemental properties. Mendeleev, however, is generally given more credit than Meyer because he published his organiz ...
... Meyer, Mendeleev, and Moseley In 1869, German chemist Lothar Meyer (1830–1895) and Russian chemist Dmitri Mendeleev (1834 –1907) each demonstrated a connection between atomic mass and elemental properties. Mendeleev, however, is generally given more credit than Meyer because he published his organiz ...
Once scientists concluded that all matter contains negatively
... approximately 66 million years ago. Proposed explanations for their extinction have ranged from an epidemic caused by some deadly microbe or virus to more gradual phenomena such as massive climate changes. In 1978 Luis Alvarez (a Nobel Prize–winning physicist), the geologist Walter Alvarez (Luis’s s ...
... approximately 66 million years ago. Proposed explanations for their extinction have ranged from an epidemic caused by some deadly microbe or virus to more gradual phenomena such as massive climate changes. In 1978 Luis Alvarez (a Nobel Prize–winning physicist), the geologist Walter Alvarez (Luis’s s ...
View PDF - CiteSeerX
... not be surprised that neither Mayer, Kelvin nor Thomson pointed out the similarity between the patterns of magnetised needles and the structure of the periodic system. Explicit references to the periodic system were rare in the 1870s, even in the chemistry literature, and only by the late 1880s had ...
... not be surprised that neither Mayer, Kelvin nor Thomson pointed out the similarity between the patterns of magnetised needles and the structure of the periodic system. Explicit references to the periodic system were rare in the 1870s, even in the chemistry literature, and only by the late 1880s had ...
Defining the Atom - Warren County Public Schools
... Teachers open the door, but you must enter by yourself. ...
... Teachers open the door, but you must enter by yourself. ...
Chapter 2: Matter Is Made up of Atoms
... thought that matter was indestructible. He made a hypothesis that all the matter present before a chemical change would still be there after the change. To find out whether a hypothesis is correct, it must be tested by repeated experiments. Lavoisier performed numerous careful experiments using diff ...
... thought that matter was indestructible. He made a hypothesis that all the matter present before a chemical change would still be there after the change. To find out whether a hypothesis is correct, it must be tested by repeated experiments. Lavoisier performed numerous careful experiments using diff ...
Boundless Study Slides
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
Chapter 3—Time and Geology
... actual geologic dating (30): The actual age, expressed in years, of a geologic material or event. alpha particle (37): A particle equivalent to the nucleus of a helium atom, emitted from an atomic nucleus during radioactive decay. Archean Eon (30): Pertaining to the division of Precambrian beginning ...
... actual geologic dating (30): The actual age, expressed in years, of a geologic material or event. alpha particle (37): A particle equivalent to the nucleus of a helium atom, emitted from an atomic nucleus during radioactive decay. Archean Eon (30): Pertaining to the division of Precambrian beginning ...
Electron - CoolHub
... be observed at various scales using models to study systems that are too large or too small. ...
... be observed at various scales using models to study systems that are too large or too small. ...
ď - Google Sites
... atom is the atom to which all the other atoms – peripheral atoms – are bonded to. Simple Lewis formulas can be predicted with a series of five steps. Determine the Lewis Formula and the structural formula for _____________________, ________________________ and _________________________. 1. Count the ...
... atom is the atom to which all the other atoms – peripheral atoms – are bonded to. Simple Lewis formulas can be predicted with a series of five steps. Determine the Lewis Formula and the structural formula for _____________________, ________________________ and _________________________. 1. Count the ...
ch05.ppt - James Goodwin
... 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make this incorrect Copyright 2012 John Wiley & Sons, Inc ...
... 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make this incorrect Copyright 2012 John Wiley & Sons, Inc ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... 1. List 20 of your favorite foods and drinks. 2. Describe basic characteristics of each of your food and drink items. For example, you might describe the primary ingredient, nutritional value, taste, and color of each item. You also could identify the food group of each item such as fruits/vegetable ...
... 1. List 20 of your favorite foods and drinks. 2. Describe basic characteristics of each of your food and drink items. For example, you might describe the primary ingredient, nutritional value, taste, and color of each item. You also could identify the food group of each item such as fruits/vegetable ...
Your Turn
... 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make this incorrect Copyright 2012 John Wiley & Sons, Inc ...
... 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make this incorrect Copyright 2012 John Wiley & Sons, Inc ...
What is the difference between atomic mass and atomic number?
... •F0J5e. 10. Atomic number atomic mass. ...
... •F0J5e. 10. Atomic number atomic mass. ...