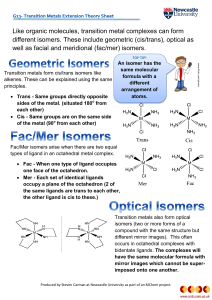
Like organic molecules, transition metal complexes can form
... Transition metals also form optical isomers (two or more forms of a compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto ...
... Transition metals also form optical isomers (two or more forms of a compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto ...
Magnetism in Transition Metal Complexes
... contribution from L (egs. Co2+ and Co3+, Fe2+ above) • non-degenerate ground states do not have a contribution from L so values close to the spin only moment are usually found (eg. Ni2+) ...
... contribution from L (egs. Co2+ and Co3+, Fe2+ above) • non-degenerate ground states do not have a contribution from L so values close to the spin only moment are usually found (eg. Ni2+) ...
CH 23 HW
... oxoanions (§23.1) 5. Why many transition metal compounds are colored and paramagnetic (§23.1) 6. The common +3 oxidation state of lanthanides and the similarity in their M3+ radii; the radioactivity of actinides (§23.2) 7. The coordination numbers, geometries, and ligand structures of complex ions ( ...
... oxoanions (§23.1) 5. Why many transition metal compounds are colored and paramagnetic (§23.1) 6. The common +3 oxidation state of lanthanides and the similarity in their M3+ radii; the radioactivity of actinides (§23.2) 7. The coordination numbers, geometries, and ligand structures of complex ions ( ...
Building Molecular Orbitals for a Square Pyramidal Oxorhenium(V
... 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s group in Organometallics 2015, 34, 3152-3158. This complex is reported to have a “distorted” square pyramidal geometry. For the purpose of this exercise, first consider the structure to be an “ideal” square pyramid of formula M(L)3 ...
... 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s group in Organometallics 2015, 34, 3152-3158. This complex is reported to have a “distorted” square pyramidal geometry. For the purpose of this exercise, first consider the structure to be an “ideal” square pyramid of formula M(L)3 ...
Activity - IONiC / VIPEr
... a. How are the orbitals of metal d character affected in your MO diagram by the absence of a ligand L on the z axis? b. Redraw your MO diagram for orbitals of metal d character in a square pyramidal complex. Label the orbitals. 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s gro ...
... a. How are the orbitals of metal d character affected in your MO diagram by the absence of a ligand L on the z axis? b. Redraw your MO diagram for orbitals of metal d character in a square pyramidal complex. Label the orbitals. 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s gro ...
Color and Bonding in Transition Metal Complexes
... Diamagnetism – All electrons are paired up, which leads to equal numbers of spin up and spin down electrons (i.e. [Co(CN)6]3-) Paramagnetism – Unpaired electrons, which leads to unequal numbers of spin up and spin down electrons ((i.e. [CoF6]3-) ...
... Diamagnetism – All electrons are paired up, which leads to equal numbers of spin up and spin down electrons (i.e. [Co(CN)6]3-) Paramagnetism – Unpaired electrons, which leads to unequal numbers of spin up and spin down electrons ((i.e. [CoF6]3-) ...
Theoretical studies on the electronic properties and
... These parameters are used then in a full CI ligand field program to calculate energies and electronic properties of all multiplets split out of a dn configuration. Symmetry analysis is supported by the program, however it is a great merit of this approach being able to calculate systems of symmetry ...
... These parameters are used then in a full CI ligand field program to calculate energies and electronic properties of all multiplets split out of a dn configuration. Symmetry analysis is supported by the program, however it is a great merit of this approach being able to calculate systems of symmetry ...
Powerpoint file (2 slides per page)
... In these complexes the color comes from absorption of light that leads to excitation of an electron from one d-orbital to a different d-orbital on the same metal cation. ...
... In these complexes the color comes from absorption of light that leads to excitation of an electron from one d-orbital to a different d-orbital on the same metal cation. ...
Chapter 7, 8, and 9 Exam 2014 Name I. 50% of your grade will come
... Chapter 7, 8, and 9 Exam 2016 ...
... Chapter 7, 8, and 9 Exam 2016 ...
A 1
... There is no combination of ligand σ orbitals with the symmetry of the metal T2g orbitals, so these do not participate in σ bonding. ...
... There is no combination of ligand σ orbitals with the symmetry of the metal T2g orbitals, so these do not participate in σ bonding. ...
TRANSITION METALS
... a liquid. Since the Enthalpy of atomization of is high it is clear that the bonding in transition metals are strong. 5. Metallic Character :- All transition elements have a) Relatively low ionization energy and b) More empty orbital than the number of electrons (one or two) in their valence shell he ...
... a liquid. Since the Enthalpy of atomization of is high it is clear that the bonding in transition metals are strong. 5. Metallic Character :- All transition elements have a) Relatively low ionization energy and b) More empty orbital than the number of electrons (one or two) in their valence shell he ...
Ground State
... Atoms and molecules (atomistic or molecular) Coupled structural elements (finite-element) Medium described by fields and distributions (continuum) Cu Crystal: potential U(r1, r2, …, rN) various properties ...
... Atoms and molecules (atomistic or molecular) Coupled structural elements (finite-element) Medium described by fields and distributions (continuum) Cu Crystal: potential U(r1, r2, …, rN) various properties ...
2015 Ch112 – problem set 5 Due: Thursday, Nov
... 2015 Ch112 – problem set 5 Due: Thursday, Nov. 19 – before class Remember to turn in your project abstract with this problem set c. Starting from your answer to part b, draw the qualitative MO diagram for the Mo-Mo-Cr interaction. Consider whether atomic orbitals/molecular orbitals will mix signifi ...
... 2015 Ch112 – problem set 5 Due: Thursday, Nov. 19 – before class Remember to turn in your project abstract with this problem set c. Starting from your answer to part b, draw the qualitative MO diagram for the Mo-Mo-Cr interaction. Consider whether atomic orbitals/molecular orbitals will mix signifi ...
Slide 1
... In these complexes the color comes from absorption of light that leads to excitation of an electron from one d-orbital to a different d-orbital on the same metal cation. ...
... In these complexes the color comes from absorption of light that leads to excitation of an electron from one d-orbital to a different d-orbital on the same metal cation. ...
chemistry 1000 - U of L Class Index
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
Lectures 31-33
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 Part-A
... Answer any eight questions. Each question carries five marks. ...
... Answer any eight questions. Each question carries five marks. ...
A 1
... Reading off the character table, we see that the group orbitals match the metal s orbital (A1g), the metal p orbitals (T1u), and the dz2 and dx2-y2 metal d orbitals (Eg). We expect bonding/antibonding combinations. The remaining three metal d orbitals are T2g and σ-nonbonding. ...
... Reading off the character table, we see that the group orbitals match the metal s orbital (A1g), the metal p orbitals (T1u), and the dz2 and dx2-y2 metal d orbitals (Eg). We expect bonding/antibonding combinations. The remaining three metal d orbitals are T2g and σ-nonbonding. ...
Exercises_Exam_III_material_2005
... Excited state term symbols for allowed term symbol for symbol for complex transitions (see note* below) free ion ...
... Excited state term symbols for allowed term symbol for symbol for complex transitions (see note* below) free ion ...
Chapter 23 – Transition Metals and Coordination Chemistry
... Complex of porphine and metal is known as porphyrin. Variations possess diff. metals, diff. groups attached to porphine. This type of complex is a component of myoglobin (stores oxygen), hemoglobin (transports oxygen in blood) and chlorophyl (needed for photosynthesis in plants). The iron in hemoglo ...
... Complex of porphine and metal is known as porphyrin. Variations possess diff. metals, diff. groups attached to porphine. This type of complex is a component of myoglobin (stores oxygen), hemoglobin (transports oxygen in blood) and chlorophyl (needed for photosynthesis in plants). The iron in hemoglo ...
Jahn–Teller effect
-3D-balls.png?width=300)
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbital nonlinear spatially degenerate molecules cannot be stable. The Jahn–Teller theorem essentially states that any nonlinear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geometrical distortion that occurs in crystals with substitutional impurities see article off-center ions.























