
Metal-Metal Bonds
... 224 pm. It was the first complex found to have a quadruple bond. Look at other complexes that have metal-metal bonds. ...
... 224 pm. It was the first complex found to have a quadruple bond. Look at other complexes that have metal-metal bonds. ...
EXAMINING THE IMPACT OF LIGAND BASICITY ON THE REACTIVITY OF TRANSITION METAL SYSTEMS THROUGH COMPUTATIONAL METHODS
... the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunately, a detailed understanding of the fundamental impact of ligand basicity on a metal c ...
... the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunately, a detailed understanding of the fundamental impact of ligand basicity on a metal c ...
Chemistry 324 Midterm 2 Name: KEY
... These CANNOT be d→d transitions because they are intense not weak. They must therefore be charge transfer transitions of some type. Cyanide is a stable anion and similar to halogens except that it has electron density in the CN pi bond. Since N is electronegative, the orbital energy of the CN pi sys ...
... These CANNOT be d→d transitions because they are intense not weak. They must therefore be charge transfer transitions of some type. Cyanide is a stable anion and similar to halogens except that it has electron density in the CN pi bond. Since N is electronegative, the orbital energy of the CN pi sys ...
Tris-2,2’-bipyridine Complexes of Iron(II) and Ruthenium (II): Synthesis, Spectroscopy and Electrochemistry
... You will probe the redox behavior of [Ru(bpy)3]2+ and [Fe(bpy)3]2+. Cyclic Voltammetry (CV) will be used to make the measurements. Make up 50 mL of acetonitrile/0.1 M TEABF4 (tetraethylammonium tetrafluoroborate), set up the electrochemical cell and bubble the solution with argon or nitrogen for app ...
... You will probe the redox behavior of [Ru(bpy)3]2+ and [Fe(bpy)3]2+. Cyclic Voltammetry (CV) will be used to make the measurements. Make up 50 mL of acetonitrile/0.1 M TEABF4 (tetraethylammonium tetrafluoroborate), set up the electrochemical cell and bubble the solution with argon or nitrogen for app ...
General Chemistry - Review for final exam: (Make sure you bring
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
... 30. How many orbitals & electrons are in the following systems? a. s b. p c. d d. f 31. What is the max # of electrons that can fit into an orbital? ...
Key Concepts PowerPoint
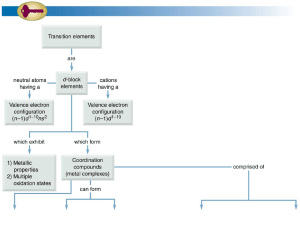
... Locate on the periodic table below the transition elements with the following electron configurations. Identify each element. (a) [Ar] 3d 7 4s2 (c) [Kr] 4d 2 5s2 ...
... Locate on the periodic table below the transition elements with the following electron configurations. Identify each element. (a) [Ar] 3d 7 4s2 (c) [Kr] 4d 2 5s2 ...
Chapter 10 Homework Problem Molecular Orbital Diagram for Cr
... 7. (3 pts) Which end of the CO ligand bonds to the metal? Looking at your answers to questions 4 and 5, is there an orbital on that atom that can serve as a σ donor to the metal? Draw a picture of this orbital. Remember: ligands use frontier molecular orbitals to bond with the metal. 8. (3 pts) Does ...
... 7. (3 pts) Which end of the CO ligand bonds to the metal? Looking at your answers to questions 4 and 5, is there an orbital on that atom that can serve as a σ donor to the metal? Draw a picture of this orbital. Remember: ligands use frontier molecular orbitals to bond with the metal. 8. (3 pts) Does ...
CHM 101
... The reactants in a chemical change have 487 kJ of energy. The change they undergo has a H = -157 kJ. The activation energy for the reaction is 570 kJ. a. Draw the energy vs reaction progress graph on the axes above paying attention to all values. Label a point that represents all products and one t ...
... The reactants in a chemical change have 487 kJ of energy. The change they undergo has a H = -157 kJ. The activation energy for the reaction is 570 kJ. a. Draw the energy vs reaction progress graph on the axes above paying attention to all values. Label a point that represents all products and one t ...
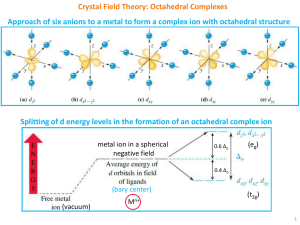
Crystal Field Theory: Octahedral Complexes
... There are only 4 ligands in the tetrahedral complex and hence the ligand field is roughly 2/3 of the octahedral field. The direction of ligand approach in tetrahedral complex does not coincide with the d-orbitals. This reduces the field by a factor of 2/3. Therefore Δt is roughly 2/3 x 2/3 = 4/9 ...
... There are only 4 ligands in the tetrahedral complex and hence the ligand field is roughly 2/3 of the octahedral field. The direction of ligand approach in tetrahedral complex does not coincide with the d-orbitals. This reduces the field by a factor of 2/3. Therefore Δt is roughly 2/3 x 2/3 = 4/9 ...
The atom in electric field
... • For the hydrogen atom – the energy levels are degenerate (except the ground state) • For the ground state the first-order perturbation correction is ...
... • For the hydrogen atom – the energy levels are degenerate (except the ground state) • For the ground state the first-order perturbation correction is ...
CH2ch24_2
... 1) A characteristic of transition metal complexes is color arising from electronic transitions between d-orbitals of different energies a) Electronic transition in an octahedral d1 complex ...
... 1) A characteristic of transition metal complexes is color arising from electronic transitions between d-orbitals of different energies a) Electronic transition in an octahedral d1 complex ...
Learning Intentions Inorganic
... o Explain the dual nature of light/radiation o Know the equations that enable the energy of a photon or of a mole of photon to be calculated o Know the name of the constants represented by h and L and where to find their values. Electron configuration and the periodic table o ...
... o Explain the dual nature of light/radiation o Know the equations that enable the energy of a photon or of a mole of photon to be calculated o Know the name of the constants represented by h and L and where to find their values. Electron configuration and the periodic table o ...
File - Science With BLT
... 1. The periodic law allows some properties of an element to be predicted based on its a. position in the periodic table. c. symbol. b. number of isotopes. d. color. 2. The periodic law states that a. no two electrons with the same spin can be found in the same place in an atom. b. the physical and c ...
... 1. The periodic law allows some properties of an element to be predicted based on its a. position in the periodic table. c. symbol. b. number of isotopes. d. color. 2. The periodic law states that a. no two electrons with the same spin can be found in the same place in an atom. b. the physical and c ...
Homework Set 7
... combining the p orbitals, the πb molecular orbital has a lower energy than the σb molecular orbital. b. Draw the Lewis dot structure of CO and assign formal charges based on the bond order from part a. In terms of electronegativity, why is this unusual? c. From b, how would CO bond to positive metal ...
... combining the p orbitals, the πb molecular orbital has a lower energy than the σb molecular orbital. b. Draw the Lewis dot structure of CO and assign formal charges based on the bond order from part a. In terms of electronegativity, why is this unusual? c. From b, how would CO bond to positive metal ...
lit_questions_VIPEr
... 8. How do the C=C bond lengths in metal-bound cyclooctadiene compare with those in free cyclooctadiene? What does this suggest about the bond order for the C=C bonds in cyclooctadiene when it coordinates to a metal center? ...
... 8. How do the C=C bond lengths in metal-bound cyclooctadiene compare with those in free cyclooctadiene? What does this suggest about the bond order for the C=C bonds in cyclooctadiene when it coordinates to a metal center? ...
collective states of 2d electron-hole system under the influence of
... The electric field strength perpendicular to the layer surface gives rise to Rashba spin-orbit coupling (RSOC)[2]. The main results of the influence of spin-orbit coupling on the 2D Wannier-Mott excitons in double quantum well structures are breaking of the spin degeneracy of the electrons and holes ...
... The electric field strength perpendicular to the layer surface gives rise to Rashba spin-orbit coupling (RSOC)[2]. The main results of the influence of spin-orbit coupling on the 2D Wannier-Mott excitons in double quantum well structures are breaking of the spin degeneracy of the electrons and holes ...
1 [L 5 FeO 2 ] - physics.muni.cz
... triplet state. The overwhelming majority of organic molecules (such as glucose or n-hexane) have all electrons paired and occur therefore in the singlet state. The products of oxidation of organic molecules, CO2 and H2O, are also in singlet states. According to the so-called Wigner-rule, processes i ...
... triplet state. The overwhelming majority of organic molecules (such as glucose or n-hexane) have all electrons paired and occur therefore in the singlet state. The products of oxidation of organic molecules, CO2 and H2O, are also in singlet states. According to the so-called Wigner-rule, processes i ...
Honors Midterm Review – 2015-16
... _________ responsible for the equation which determines the exact amount of energy needed for electron promotion or demotion between energy levels _________ responsible for the idea that if light can behave as particles of matter, matter should also be able to exhibit wave properties. ...
... _________ responsible for the equation which determines the exact amount of energy needed for electron promotion or demotion between energy levels _________ responsible for the idea that if light can behave as particles of matter, matter should also be able to exhibit wave properties. ...
Problems - UCI Chemistry
... 10.39 Calculate and view the molecular orbitals of the octahedral ion [TiF6]3 - . a. Identify the t2g and eg bonding and antibonding orbitals, and indicate which d orbitals of Ti are involved in each. b. Compare your results with Figures 10.5 and 10.7. Do they indicate that fluoride is acting as a p ...
... 10.39 Calculate and view the molecular orbitals of the octahedral ion [TiF6]3 - . a. Identify the t2g and eg bonding and antibonding orbitals, and indicate which d orbitals of Ti are involved in each. b. Compare your results with Figures 10.5 and 10.7. Do they indicate that fluoride is acting as a p ...
Chapter 1 Structure and Bonding
... Unequal occupation of degenerate orbitals is forbidden 1) To obey this theorem, metal complexes with offending electronic structures must distort to “break” the degeneracy ...
... Unequal occupation of degenerate orbitals is forbidden 1) To obey this theorem, metal complexes with offending electronic structures must distort to “break” the degeneracy ...
Jahn–Teller effect
-3D-balls.png?width=300)
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbital nonlinear spatially degenerate molecules cannot be stable. The Jahn–Teller theorem essentially states that any nonlinear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geometrical distortion that occurs in crystals with substitutional impurities see article off-center ions.
















![1 [L 5 FeO 2 ] - physics.muni.cz](http://s1.studyres.com/store/data/000263887_1-9a7fea8feae8a4c4c33cd53b2038de6b-300x300.png)



