Define:
... 42. In the number 0.305 L, which digit is estimated? 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi ...
... 42. In the number 0.305 L, which digit is estimated? 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi ...
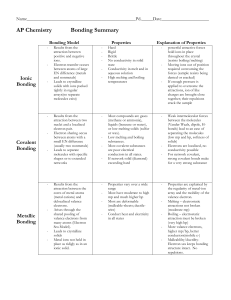
Types of Bonding Summary
... (malleable-sheets; ductilewire) Conduct heat and electricity in all states ...
... (malleable-sheets; ductilewire) Conduct heat and electricity in all states ...
BASIC CHEMISTRY
... number of protons and neutrons in the nucleus and the electrons in the energy level. ...
... number of protons and neutrons in the nucleus and the electrons in the energy level. ...
Review Sheet Filled Out
... Can’t tell where an electron is at any moment in time – the uncertainty principle There’s more – the list could be long! ...
... Can’t tell where an electron is at any moment in time – the uncertainty principle There’s more – the list could be long! ...
Section 1 Atoms, Elements, and Compounds
... Polar- unequal sharing of electrons. Creates dipoles. Example: water Non polar-equal sharing. Example: methane Ionic One atom obtains (steals) one or more electrons from another atom. Creates ions. Charges between ions create the bond. Strong force. Example: Salt (NaCl) Section 2: Ch ...
... Polar- unequal sharing of electrons. Creates dipoles. Example: water Non polar-equal sharing. Example: methane Ionic One atom obtains (steals) one or more electrons from another atom. Creates ions. Charges between ions create the bond. Strong force. Example: Salt (NaCl) Section 2: Ch ...
worksheet 7b answers - Iowa State University
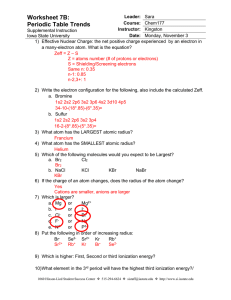
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Chapter 9: Chemical Quantities
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
Chemistry of Life - juan-roldan
... atomic mass of an atom ◦ Is a number that indicates how much matter it contains. ◦ Is expressed by the atomic mass unit (amu), also known as the dalton. ◦ The atomic mass= number of protons + number of neutrons ...
... atomic mass of an atom ◦ Is a number that indicates how much matter it contains. ◦ Is expressed by the atomic mass unit (amu), also known as the dalton. ◦ The atomic mass= number of protons + number of neutrons ...
Chapter 5
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
Review for second exam:
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
Course Syllabus - Honors Chemistry
... e. Draw Lewis dot structures. f.* Predict the shape and polarity of simple molecules from Lewis dot structures. g.* Electronegativity and ionization energy relate to bond formation. h.* Identify solids and liquids held together by Van der Waals forces or hydrogen bonding and relate these forces to v ...
... e. Draw Lewis dot structures. f.* Predict the shape and polarity of simple molecules from Lewis dot structures. g.* Electronegativity and ionization energy relate to bond formation. h.* Identify solids and liquids held together by Van der Waals forces or hydrogen bonding and relate these forces to v ...
Review Notes - Biochemistry
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
ExamView - Untitled.tst
... ____ 13. Thomson made his discovery about the atom during an experiment using a. thermal energy. c. cathode rays b. kinetic energy. d. X rays. ____ 14. In _____ atomic model, negative electrons orbit the positively charged nucleus. a. Dalton’s c. Rutherford’s b. Thomson’s d. Democritus’s ____ 15. Wh ...
... ____ 13. Thomson made his discovery about the atom during an experiment using a. thermal energy. c. cathode rays b. kinetic energy. d. X rays. ____ 14. In _____ atomic model, negative electrons orbit the positively charged nucleus. a. Dalton’s c. Rutherford’s b. Thomson’s d. Democritus’s ____ 15. Wh ...
Notes 4.1 In the fourth century BC, the Greek philosopher
... that the universe was made of indivisible units. This suggestion did not have any evidence. As thinkers and technology improved more data was collected and theories were developed. Daltons Atomic Theory stated that all atoms of a given element were exactly alike, and atoms of different elements coul ...
... that the universe was made of indivisible units. This suggestion did not have any evidence. As thinkers and technology improved more data was collected and theories were developed. Daltons Atomic Theory stated that all atoms of a given element were exactly alike, and atoms of different elements coul ...
Metastable inner-shell molecular state

Metastable Innershell Molecular State (MIMS) is a class of ultra-high-energy short-lived molecules have the binding energy up to 1,000 times larger and bond length up to 100 times smaller than typical molecules. MIMS is formed by inner-shell electrons that are normally resistant to molecular formation. However, in stellar conditions, the inner-shell electrons become reactive to form molecular structures (MIMS) from combinations of all elements in the periodic table. MIMS upon dissociation can emit x-ray photons with energies up to 100 keV at extremely high conversion efficiencies from compression energy to photon energy. MIMS is predicted to exist and dominate radiation processes in extreme astrophysical environments, such as large planet cores, star interiors, and black hole and neutron star surroundings. There, MIMS is predicted to enable highly energy-efficient transformation of the stellar compression energy into the radiation energy.The right schematic illustration shows the proposed four stages of the K-shell MIMS (K-MIMS) formation and x-ray generation process. Stage I: Individual atoms are subjected to the stellar compression and ready for absorbing the compression energy. Stage II: The outer electron shells fuse together under increasing ""stellar"" pressure. Stage III: At the peak pressure, via pressure ionization K-shell orbits form the K-MIMS, which is vibrationally hot and encapsulated by a Rydberg-like pseudo-L-Shell structure. Stage IV: The K-MIMS cools down by ionizing (""boiling-off"") a number of pseudo-L-shell electrons and subsequent optical decay by emitting an x-ray photon. The dissociated atoms return their original atoms states and are ready for absorbing the compression energy.MIMS also can be readily produced in laboratory and industrial environments, such as hypervelocity particle impact, laser fusion and z-machine. MIMS can be exploited for highly energy-efficient production of high intensity x-ray beams for a wide range of innovative applications, such as photolithography, x-ray lasers, and inertial fusion.