Fitting X-ray Spectra with Imperfect Models
... Line Emission from Collisional Excitation or something else more exotic? In the “coronal” limit the electron density Ne is low enough that most of the level population is in the ground state. Flux = ∑ ε (Te) EM(Te) / (4 π R2), where R is the distance, EM (Te) = ∫ Ne NH dV is the emission measure, a ...
... Line Emission from Collisional Excitation or something else more exotic? In the “coronal” limit the electron density Ne is low enough that most of the level population is in the ground state. Flux = ∑ ε (Te) EM(Te) / (4 π R2), where R is the distance, EM (Te) = ∫ Ne NH dV is the emission measure, a ...
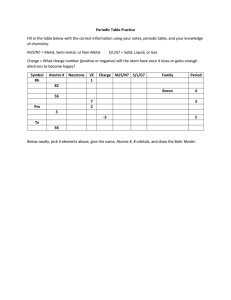
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 15. A photon with some energy Ep strikes a metal surface. An electron is ejected with a velocity of 5.00 Mm/s. The threshold frequency of the metal is 6.30 x 1013 s-1. What is the wavelength of the photon, in nm? Significant figures count. 17 nm ...
... 15. A photon with some energy Ep strikes a metal surface. An electron is ejected with a velocity of 5.00 Mm/s. The threshold frequency of the metal is 6.30 x 1013 s-1. What is the wavelength of the photon, in nm? Significant figures count. 17 nm ...
topic 1 sol review homework
... a) more protons b) less protons c) more neutrons d) less neutrons 25. How are the bright-line spectrum of elements produced? when electrons go from a higher energy level back to the ground state releaseing enegy in the form of visible light 26. When at atom goes from the excited state to the ground ...
... a) more protons b) less protons c) more neutrons d) less neutrons 25. How are the bright-line spectrum of elements produced? when electrons go from a higher energy level back to the ground state releaseing enegy in the form of visible light 26. When at atom goes from the excited state to the ground ...
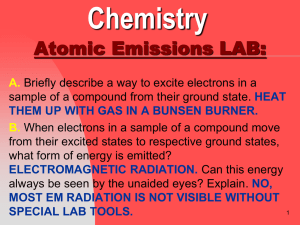
Slide 1
... D(DG) = -RTefflnkeq = -RTeffln(k3/k2) = RTeffln(k2/k3) The ratio is equal to the product ratio, which is to a good approximation equal to the ratio of the intensities for these two ions in the MS/MS spectrum: ...
... D(DG) = -RTefflnkeq = -RTeffln(k3/k2) = RTeffln(k2/k3) The ratio is equal to the product ratio, which is to a good approximation equal to the ratio of the intensities for these two ions in the MS/MS spectrum: ...
2DCAtStrUT2010
... 11. Why are most masses on the Periodic Table decimal values instead of whole numbers? Answer: _____________________________________________________________________________ 12. As an atom is ionized, its number of protons... a) decreases b) increases c) decreases or increases d) doesn’t change 13. W ...
... 11. Why are most masses on the Periodic Table decimal values instead of whole numbers? Answer: _____________________________________________________________________________ 12. As an atom is ionized, its number of protons... a) decreases b) increases c) decreases or increases d) doesn’t change 13. W ...
Nanotechnology: From Microelectronics to Health Care
... molecular ion is observed in EI mass spectrum, and also in the case of confirming the mass to charge ratio of the molecular ion. -Chemical ionization technique uses virtually the same ion source device as in electron impact, except, CI uses tight ion source, and reagent gas. Reagent gas (e.g. ammoni ...
... molecular ion is observed in EI mass spectrum, and also in the case of confirming the mass to charge ratio of the molecular ion. -Chemical ionization technique uses virtually the same ion source device as in electron impact, except, CI uses tight ion source, and reagent gas. Reagent gas (e.g. ammoni ...
File - Ingolstadt Academy
... Dimensional analysis Instruments that measure mass, volume, pressure, etc. (lab stuff!) The Scientific Method Atomic Structure: ...
... Dimensional analysis Instruments that measure mass, volume, pressure, etc. (lab stuff!) The Scientific Method Atomic Structure: ...
SUMMER WORK AP Chemistry
... 16. (a) Calculate the energy of a photon of electromagnetic radiation whose frequency is 6.74 x 1012 s-1. (b) Calculate the energy of a photon of radiation whose wavelength is 322 nm. (c) What wavelength of radiation has photons of energy 2.87 x 10-18 J. 17. Bohr’s model can be used for hydrogen-li ...
... 16. (a) Calculate the energy of a photon of electromagnetic radiation whose frequency is 6.74 x 1012 s-1. (b) Calculate the energy of a photon of radiation whose wavelength is 322 nm. (c) What wavelength of radiation has photons of energy 2.87 x 10-18 J. 17. Bohr’s model can be used for hydrogen-li ...
Unit 16 Worksheet - Jensen Chemistry
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
Unit 4 Compounds, Naming, Formula Writing
... Whenever 2 elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. ...
... Whenever 2 elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. ...
powerpoint version - Leeds Astrophysics
... Brahe made very accurate observations to one minute of arc (1’ - there are 60 minutes in a degree and 360 degrees in a full circle) Kepler was a mathematician who used Brahe’s data to develop a heliocentric model. He used the concept of a force between the Sun and planets but did not quantify it. PH ...
... Brahe made very accurate observations to one minute of arc (1’ - there are 60 minutes in a degree and 360 degrees in a full circle) Kepler was a mathematician who used Brahe’s data to develop a heliocentric model. He used the concept of a force between the Sun and planets but did not quantify it. PH ...
Define:
... 71. What is the frequency of ultraviolet light with a wavelength of 4.92 x 10-8m? 72. What is the wavelength of a gamma ray with a frequency of 3.72 x 1020Hz? 73. If three electrons are available to fill three empty 2p orbitals, how will the electrons be distributed? 74. Stable electron configuratio ...
... 71. What is the frequency of ultraviolet light with a wavelength of 4.92 x 10-8m? 72. What is the wavelength of a gamma ray with a frequency of 3.72 x 1020Hz? 73. If three electrons are available to fill three empty 2p orbitals, how will the electrons be distributed? 74. Stable electron configuratio ...
Basic Atomic Theory
... • Strength of Coulomb forces much larger than gravitational • +ve and –ve charges cause attractive and repulsive interactions. ...
... • Strength of Coulomb forces much larger than gravitational • +ve and –ve charges cause attractive and repulsive interactions. ...
Metastable inner-shell molecular state

Metastable Innershell Molecular State (MIMS) is a class of ultra-high-energy short-lived molecules have the binding energy up to 1,000 times larger and bond length up to 100 times smaller than typical molecules. MIMS is formed by inner-shell electrons that are normally resistant to molecular formation. However, in stellar conditions, the inner-shell electrons become reactive to form molecular structures (MIMS) from combinations of all elements in the periodic table. MIMS upon dissociation can emit x-ray photons with energies up to 100 keV at extremely high conversion efficiencies from compression energy to photon energy. MIMS is predicted to exist and dominate radiation processes in extreme astrophysical environments, such as large planet cores, star interiors, and black hole and neutron star surroundings. There, MIMS is predicted to enable highly energy-efficient transformation of the stellar compression energy into the radiation energy.The right schematic illustration shows the proposed four stages of the K-shell MIMS (K-MIMS) formation and x-ray generation process. Stage I: Individual atoms are subjected to the stellar compression and ready for absorbing the compression energy. Stage II: The outer electron shells fuse together under increasing ""stellar"" pressure. Stage III: At the peak pressure, via pressure ionization K-shell orbits form the K-MIMS, which is vibrationally hot and encapsulated by a Rydberg-like pseudo-L-Shell structure. Stage IV: The K-MIMS cools down by ionizing (""boiling-off"") a number of pseudo-L-shell electrons and subsequent optical decay by emitting an x-ray photon. The dissociated atoms return their original atoms states and are ready for absorbing the compression energy.MIMS also can be readily produced in laboratory and industrial environments, such as hypervelocity particle impact, laser fusion and z-machine. MIMS can be exploited for highly energy-efficient production of high intensity x-ray beams for a wide range of innovative applications, such as photolithography, x-ray lasers, and inertial fusion.