Introduction to Computational Chemistry Laboratory
... the energy associated with bond stretching, bending, rotation and intermolecular forces, such as Van der Waals interactions and hydrogen bonding. All of the constants in these equations must be obtained from experimental data or an ab-initio calculation. In a molecular mechanics method, the database ...
... the energy associated with bond stretching, bending, rotation and intermolecular forces, such as Van der Waals interactions and hydrogen bonding. All of the constants in these equations must be obtained from experimental data or an ab-initio calculation. In a molecular mechanics method, the database ...
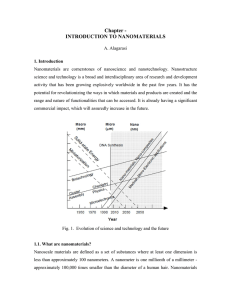
Chapter - INTRODUCTION TO NANOMATERIALS
... The interest in this synthesis method arises due to the possibility of synthesizing nonmetallic inorganic materials like glasses, glass ceramics or ceramic materials at very low temperatures compared to the high temperature process required by melting glass or firing ceramics. The major difficulties ...
... The interest in this synthesis method arises due to the possibility of synthesizing nonmetallic inorganic materials like glasses, glass ceramics or ceramic materials at very low temperatures compared to the high temperature process required by melting glass or firing ceramics. The major difficulties ...
SCHOOL OF CHEMICAL SCIENCES
... Students are required to select either KUE 309/6 or 6 units of other courses to fulfill the 65 units of Basic/Core Courses *All the courses offered are subjected to the alteration/modification when the need arises. (s) = sequential (Course must be taken earlier) (c) = concurrent (Course can be taken ...
... Students are required to select either KUE 309/6 or 6 units of other courses to fulfill the 65 units of Basic/Core Courses *All the courses offered are subjected to the alteration/modification when the need arises. (s) = sequential (Course must be taken earlier) (c) = concurrent (Course can be taken ...
Chapter 4: Oxidation and Reduction MH5 4
... Unit 3 Oxidation and Reduction Chemistry 020, R. R. Martin 1 Introduction Another important type of reaction in aqueous solution involves the transfer of electrons between two species. This is called an oxidation-reduction or a redox reaction. What happens when zinc pellets are added to an acid? The ...
... Unit 3 Oxidation and Reduction Chemistry 020, R. R. Martin 1 Introduction Another important type of reaction in aqueous solution involves the transfer of electrons between two species. This is called an oxidation-reduction or a redox reaction. What happens when zinc pellets are added to an acid? The ...
The Chemist - American Institute of Chemists
... revolution in ‘chemical practice’ is further evidence of chemistry and the chemical industry making a continuing commitment to recognise and address its responsibilities to society at large. The general populace has a fear of chemistry because it does not understand its language or the models that a ...
... revolution in ‘chemical practice’ is further evidence of chemistry and the chemical industry making a continuing commitment to recognise and address its responsibilities to society at large. The general populace has a fear of chemistry because it does not understand its language or the models that a ...
Example 1-2
... The textbook we will be using is, Chemistry, 6th edition, by Zumdahl. I recommend picking up a copy from ebay or amazon so that you have you own personal copy in which you can write and take notes. However, the school will also be providing you with a copy on the first day of class. The final part o ...
... The textbook we will be using is, Chemistry, 6th edition, by Zumdahl. I recommend picking up a copy from ebay or amazon so that you have you own personal copy in which you can write and take notes. However, the school will also be providing you with a copy on the first day of class. The final part o ...
chemistry - Mount Holyoke College Catalog
... of high school chemistry. CHEM-101 provides such a student with an opportunity to develop her understanding of the foundations of reaction chemistry, thermochemistry, electronic structure, chemical bonding, and acid-base chemistry. Students interested in studying biochemistry, or interested in satis ...
... of high school chemistry. CHEM-101 provides such a student with an opportunity to develop her understanding of the foundations of reaction chemistry, thermochemistry, electronic structure, chemical bonding, and acid-base chemistry. Students interested in studying biochemistry, or interested in satis ...
13.0 Redox Reactions PowerPoint
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
Chapter 19: Thermochemistry II: Entropy and free Energy
... in an automobile, concentrated chemical energy in the form of fossil fuel is converted into hot gases (thermal energy) that drive pistons (mechanical energy) and are then spewed out of the tail pipe and cool in the surrounding atmosphere (lower temperature thermal energy). ...
... in an automobile, concentrated chemical energy in the form of fossil fuel is converted into hot gases (thermal energy) that drive pistons (mechanical energy) and are then spewed out of the tail pipe and cool in the surrounding atmosphere (lower temperature thermal energy). ...
Periodic Table and the Atom Answers
... do this reaction with 125 grams of acetic acid and 275 grams of aluminum hydroxide. Two calculations are required. One determines the quantity of aluminum acetate that can be made with 125 grams of acetic acid and the other determines the quantity of aluminum acetate that can be made using 275 grams ...
... do this reaction with 125 grams of acetic acid and 275 grams of aluminum hydroxide. Two calculations are required. One determines the quantity of aluminum acetate that can be made with 125 grams of acetic acid and the other determines the quantity of aluminum acetate that can be made using 275 grams ...
www.xtremepapers.net
... bold type. A full Advanced Level qualification requires the study of further core material together with section 11, Applications of Chemistry. The Applications of Chemistry section represents about 12% of the full Advanced Level course or 23% of the A2 course. Candidates can take the course either ...
... bold type. A full Advanced Level qualification requires the study of further core material together with section 11, Applications of Chemistry. The Applications of Chemistry section represents about 12% of the full Advanced Level course or 23% of the A2 course. Candidates can take the course either ...
chapter4-bur.2917051..
... because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive or negative charge. The reason these partial charges exist will be discussed later in the semester. Because cations and ani ...
... because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive or negative charge. The reason these partial charges exist will be discussed later in the semester. Because cations and ani ...
www.xtremepapers.net
... 16 Compound X on refluxing with aqueous sodium hydroxide gave mixture Y which on distillation with acidified potassium dichromate(VI) produced propanone. Mixing Y with dilute nitric acid and aqueous silver nitrate gave a cream precipitate. What could compound X be? A ...
... 16 Compound X on refluxing with aqueous sodium hydroxide gave mixture Y which on distillation with acidified potassium dichromate(VI) produced propanone. Mixing Y with dilute nitric acid and aqueous silver nitrate gave a cream precipitate. What could compound X be? A ...
L-11 Chemical thermodynamics
... System is the part of the physical universe which is under study, while the rest of the universe is surroundings. You know that hot tea/milk (let us call it a system) kept in a stoppered thermos flask remains hot for a couple of hours. If this flask is made of perfect insulating material, then there ...
... System is the part of the physical universe which is under study, while the rest of the universe is surroundings. You know that hot tea/milk (let us call it a system) kept in a stoppered thermos flask remains hot for a couple of hours. If this flask is made of perfect insulating material, then there ...
word - My eCoach
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
Chemistry Review 2 answer key
... State evidence from the balanced equation for the cell with iron and copper electrodes that indicates the reaction in the cell is an oxidation-reduction reaction. [1] In an oxidation-reduction reaction, changes in oxidation states are observed. In this reaction, Cu changes from Cu2+ to Cu0, and Fe c ...
... State evidence from the balanced equation for the cell with iron and copper electrodes that indicates the reaction in the cell is an oxidation-reduction reaction. [1] In an oxidation-reduction reaction, changes in oxidation states are observed. In this reaction, Cu changes from Cu2+ to Cu0, and Fe c ...
2007_UG - St.Joseph`s College
... * To provide mobility and flexibility for students within and outside the parent department * To provide broad based education * To help students learn at their own pace * To provide students scope for acquiring extra credits * To impart more job oriented skills to students * To make any course mult ...
... * To provide mobility and flexibility for students within and outside the parent department * To provide broad based education * To help students learn at their own pace * To provide students scope for acquiring extra credits * To impart more job oriented skills to students * To make any course mult ...
7.1 CHEMICAL SYSTEMS IN EQUILIBRIUM: Dynamic Equilibrium in
... Explaining the conditions The proportions of nitrogen and hydrogen The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers ...
... Explaining the conditions The proportions of nitrogen and hydrogen The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers ...
Chapter 15 Acids & Bases
... Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) 3. Acids react with bases to produce salts and water: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) ...
... Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) 3. Acids react with bases to produce salts and water: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) ...