2)](http://s1.studyres.com/store/data/015968611_1-56df287e8435abc2be6b0a2948d2417f-300x300.png)
([Cu(NH3)4](MnO4)2)
... The appearance of IR-inactive Cu N stretching modes in the IR spectrum of 1 shows the symmetry-lowering due to distortion of the regular square-planar CuN4 geometry. The splittings of nÄs , ds, and 1r N H bands or the nÄas Cu N band confirm the symmetry-lowering of the complex cation. The presence o ...
... The appearance of IR-inactive Cu N stretching modes in the IR spectrum of 1 shows the symmetry-lowering due to distortion of the regular square-planar CuN4 geometry. The splittings of nÄs , ds, and 1r N H bands or the nÄas Cu N band confirm the symmetry-lowering of the complex cation. The presence o ...
ordinary level chemistry syllabus
... 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed significantly to the global socio-economic transformation. This level of contribution has been achieved through the range of important life changing discoveries by chemists. T ...
... 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed significantly to the global socio-economic transformation. This level of contribution has been achieved through the range of important life changing discoveries by chemists. T ...
THE MOLE (pp. 159
... 2. But, they do not always tell exactly how many atoms of each element are present in a molecule of the compound. For that one needs the _______________________________. 3. Molecular formula – a formula that gives the type and actual number of atoms in a chemical compound. 4. The molecular formula c ...
... 2. But, they do not always tell exactly how many atoms of each element are present in a molecule of the compound. For that one needs the _______________________________. 3. Molecular formula – a formula that gives the type and actual number of atoms in a chemical compound. 4. The molecular formula c ...
Chemistry
... advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built into the original work. This new edition has been extensively revised to take ...
... advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built into the original work. This new edition has been extensively revised to take ...
ism ismismismismismrapidrevisionquestionsismismismismismism
... (iii) If a reaction of higher order follows first order kinetics under special conditions, it is called pseudo first order reaction. 17. A first order reaction has a rate constant of 0.0051min-1. If we begin with 0.10M concentration of the reactant, what concentration of reactant will remain in solu ...
... (iii) If a reaction of higher order follows first order kinetics under special conditions, it is called pseudo first order reaction. 17. A first order reaction has a rate constant of 0.0051min-1. If we begin with 0.10M concentration of the reactant, what concentration of reactant will remain in solu ...
Practice Exam 4
... which as 4 orientations, we can use the Boltzmann equation to calculate the residual entropy. S = Na k ln W = R ln 4 = 11.5 J K−1 ...
... which as 4 orientations, we can use the Boltzmann equation to calculate the residual entropy. S = Na k ln W = R ln 4 = 11.5 J K−1 ...
15.0 EquilibriumIHS2014
... 1.2K: Identify, write and interpret chemical equations for systems at equilibrium 1.4K: Define Kc to predict the extent of the reaction and write equilibrium-law expressions for given chemical equations, using lowest whole-number coefficients ...
... 1.2K: Identify, write and interpret chemical equations for systems at equilibrium 1.4K: Define Kc to predict the extent of the reaction and write equilibrium-law expressions for given chemical equations, using lowest whole-number coefficients ...
2015 International Practice Exam: Chemistry
... never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose these questions through any means, your AP Exam score will be canceled. . . . You must complete the answer sheet using a No. 2 pencil only. Mark all ...
... never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose these questions through any means, your AP Exam score will be canceled. . . . You must complete the answer sheet using a No. 2 pencil only. Mark all ...
2015 chemistry
... high temperatures. _______________________________________________________________________________________________________ _______________________________________________________________________________________________________ _________________________________________________________________________ ...
... high temperatures. _______________________________________________________________________________________________________ _______________________________________________________________________________________________________ _________________________________________________________________________ ...
A Few Things You Might Want To Know
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
Semester 4 - Vaal University of Technology
... WIL is an integral part of the training and, together with University Training, form a co-operative training unit. It is therefore the aim of WIL to compel the students in his/her work situation, to be actively engaged in the broadening of his/her knowledge and analytical skills. It is also importan ...
... WIL is an integral part of the training and, together with University Training, form a co-operative training unit. It is therefore the aim of WIL to compel the students in his/her work situation, to be actively engaged in the broadening of his/her knowledge and analytical skills. It is also importan ...
09_Lecture
... direction up to a point when some of the products revert back to reactants. • A chemical equilibrium is established when forward and reverse reactions occur at the same rate. • The equilibrium constant, K, defines the extent of a chemical reaction as a ratio of the concentration of the products to t ...
... direction up to a point when some of the products revert back to reactants. • A chemical equilibrium is established when forward and reverse reactions occur at the same rate. • The equilibrium constant, K, defines the extent of a chemical reaction as a ratio of the concentration of the products to t ...
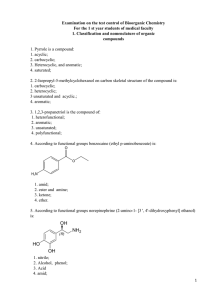
2. 2-Isopropyl-5-methylcyclohexanol on carbon skeletal
... 4 3-bromo-2-methylpentane; 52. The chlorination reaction of following compounds proceeds by a mechanism of radical substitution when exposed ultraviolet radiation: 1. cyclohexane; 2. benzene; 3. acetylene; 4. 1,3-butadiene 53. The characteristic reaction of alkenes is proceeding following mechanism ...
... 4 3-bromo-2-methylpentane; 52. The chlorination reaction of following compounds proceeds by a mechanism of radical substitution when exposed ultraviolet radiation: 1. cyclohexane; 2. benzene; 3. acetylene; 4. 1,3-butadiene 53. The characteristic reaction of alkenes is proceeding following mechanism ...
Chemical Equilibrium - Chemistry with Mrs. Caruso Let the Bonding
... Ex. A solution contains 1.0 x 10-4 M Cu+ and 2.0 x 10-3 M Pb2+. If a source of I- is added to this solution gradually, will PbI2 (Ksp= 1.4 x 10-8) or CuI (Ksp= 5.3x 10-12) precipitate first? What concentrations of I- are necessary to begin precipitation? PbI2 2Cl- + Pb2+ 1.4 x 10-8 = (2.0 x 10-3) ...
... Ex. A solution contains 1.0 x 10-4 M Cu+ and 2.0 x 10-3 M Pb2+. If a source of I- is added to this solution gradually, will PbI2 (Ksp= 1.4 x 10-8) or CuI (Ksp= 5.3x 10-12) precipitate first? What concentrations of I- are necessary to begin precipitation? PbI2 2Cl- + Pb2+ 1.4 x 10-8 = (2.0 x 10-3) ...
Prebiotic synthesis from CO atmospheres: Implications for the
... gen (350 Torr; 99.8% 15N, Shoko, Tokyo) was enclosed in a glass tube (400 ml) containing liquid water (5 ml). 15N2 was used to identify possible contamination. The gas mixture was irradiated with protons generated by a van de Graaff accelerator (Tokyo Institute of Technology, Tokyo) at 297 K for 3 h ...
... gen (350 Torr; 99.8% 15N, Shoko, Tokyo) was enclosed in a glass tube (400 ml) containing liquid water (5 ml). 15N2 was used to identify possible contamination. The gas mixture was irradiated with protons generated by a van de Graaff accelerator (Tokyo Institute of Technology, Tokyo) at 297 K for 3 h ...
N Goalby chemrevise.org 1 2.5 Transition Metals Substitution
... Why is Zn not a transition metal? Zn can only form a +2 ion. In this ion the Zn 2+ has a complete d orbital and so does not meet the criteria of having an incomplete d orbital in one of its compounds. ...
... Why is Zn not a transition metal? Zn can only form a +2 ion. In this ion the Zn 2+ has a complete d orbital and so does not meet the criteria of having an incomplete d orbital in one of its compounds. ...
Stoichiometry, Lab Basics, Reactions
... Aluminum reacts with hydrochloric acid, as indicated in the equation above, to produce hydrogen gas. The H2 produced was then collected by water displacement at 27C (where the vapor pressure of water is 21 torr) and a barometric pressure of 757 torr. If 0.555 L of gas is collected, the partial pre ...
... Aluminum reacts with hydrochloric acid, as indicated in the equation above, to produce hydrogen gas. The H2 produced was then collected by water displacement at 27C (where the vapor pressure of water is 21 torr) and a barometric pressure of 757 torr. If 0.555 L of gas is collected, the partial pre ...
Personal Tutoring Help on Questions and Problems
... Atomic Mass Review Questions: 3.1 – 3.4 (page 107) Problems: 3.5 – 3.8 (page 107) Avogadro’s Number and Molar Mass Review Questions: 3.9 – 3.10 (page 107) Problems: 3.11 – 3.22 (page 107) Molecular Mass Problems: 3.23 – 3.30 (page 108) ...
... Atomic Mass Review Questions: 3.1 – 3.4 (page 107) Problems: 3.5 – 3.8 (page 107) Avogadro’s Number and Molar Mass Review Questions: 3.9 – 3.10 (page 107) Problems: 3.11 – 3.22 (page 107) Molecular Mass Problems: 3.23 – 3.30 (page 108) ...