Document
... 21. If 2.891 g MgCl2 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the magnesium chloride solution? ...
... 21. If 2.891 g MgCl2 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the magnesium chloride solution? ...
Chemistry - Ysgol Bro Pedr
... You will find that several reactions involve ions in solution. In these reactions, not all of the ions actually take part in the reaction, they just seem to float around doing nothing and are known as ‘spectator ions’. If you take these ions out of the equation, you end up with an ionic equation, wh ...
... You will find that several reactions involve ions in solution. In these reactions, not all of the ions actually take part in the reaction, they just seem to float around doing nothing and are known as ‘spectator ions’. If you take these ions out of the equation, you end up with an ionic equation, wh ...
chemistry-subject test5 w. solutions
... dispersion forces (in the order of decreasing strength). For the ideal gas law to give an accurate prediction of the volume, then, we are looking for gases that do not have ahttp://doc.guandang.net/bbca35c11081d34250955e480.html strong dipole moment. Methane, CH4, does not have a dipole moment: What ...
... dispersion forces (in the order of decreasing strength). For the ideal gas law to give an accurate prediction of the volume, then, we are looking for gases that do not have ahttp://doc.guandang.net/bbca35c11081d34250955e480.html strong dipole moment. Methane, CH4, does not have a dipole moment: What ...
chemistry paper 1
... An experiment was performed on the study of the rate of reaction between hydrochloric acid and sodium thiosulphate solution. 10 cm3 portions of 2.0 M hydrochloric acid were added to four separate conical flasks, W, X, Y and Z, each containing sodium thiosulphate solution which was prepared respectiv ...
... An experiment was performed on the study of the rate of reaction between hydrochloric acid and sodium thiosulphate solution. 10 cm3 portions of 2.0 M hydrochloric acid were added to four separate conical flasks, W, X, Y and Z, each containing sodium thiosulphate solution which was prepared respectiv ...
Chemistry and Biochemistry - St. Mary`s University Academic Catalog
... This course is designed to provide a general overview of these two specific areas of chemistry for non- majors. It will provide the general basics of organic chemistry including basic carbon chemistry, nomenclature, structures of organic compounds, chemical characteristics and function,reactions, an ...
... This course is designed to provide a general overview of these two specific areas of chemistry for non- majors. It will provide the general basics of organic chemistry including basic carbon chemistry, nomenclature, structures of organic compounds, chemical characteristics and function,reactions, an ...
CHE 1031 Lab Manual
... corrosivity, reactivity or toxicity. In order to protect yourself from the chemicals you use in the chemistry laboratory, it is important to understand and be aware of these hazards. In addition, essential ...
... corrosivity, reactivity or toxicity. In order to protect yourself from the chemicals you use in the chemistry laboratory, it is important to understand and be aware of these hazards. In addition, essential ...
Surface chemistry and Catalysis
... level off near the saturation vapour pressure and are considered to reflect capillary condensation in porous solids, the effective pore diameters usually being between 2 nm and 20 nm. The upper limit of adsorption is mainly governed by the total pore volume. ...
... level off near the saturation vapour pressure and are considered to reflect capillary condensation in porous solids, the effective pore diameters usually being between 2 nm and 20 nm. The upper limit of adsorption is mainly governed by the total pore volume. ...
5. Coenzyme HAD+ is derived
... training. Chemistry is a basic science and a powerful tool for studying and learning processes in living systems. Therefore, medical students must thoroughly understand the basic ideas, laws and methods of this science. Program expected to consider the foundations of the most important topics of the ...
... training. Chemistry is a basic science and a powerful tool for studying and learning processes in living systems. Therefore, medical students must thoroughly understand the basic ideas, laws and methods of this science. Program expected to consider the foundations of the most important topics of the ...
Thin-Layer Chromatography: Applying TLC as a
... interactions of the salicylic molecules with each other in the reaction mixture. This may be because the hydrogen on the phenolic substituent of salicylic acid interacts with the double bonded oxygen of the other substituent, carboxylic acid, through hydrogen bonding thus decreasing its polarity sig ...
... interactions of the salicylic molecules with each other in the reaction mixture. This may be because the hydrogen on the phenolic substituent of salicylic acid interacts with the double bonded oxygen of the other substituent, carboxylic acid, through hydrogen bonding thus decreasing its polarity sig ...
WJEC Eduqas A Level Chemistry specification
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
electrochemistry
... exothermic reaction is normally lost as heat. However, it can be trapped and converted into electrical energy if the reactants involved are not in direct contact with each other. In galvanic cells redox reactions is split into two halfreactions, each occurring in two separate compartments, called ha ...
... exothermic reaction is normally lost as heat. However, it can be trapped and converted into electrical energy if the reactants involved are not in direct contact with each other. In galvanic cells redox reactions is split into two halfreactions, each occurring in two separate compartments, called ha ...
Chemical Engineering Principles of CVD Processes
... Mismatch in the thermal expansion coefficients between the substrate and the coating may cause poor adhesion - due to the generation of thermal stress Usually the residual stress pattern can be favorably changed by changing the deposition conditions b. Formation of brittle intermetallic compounds an ...
... Mismatch in the thermal expansion coefficients between the substrate and the coating may cause poor adhesion - due to the generation of thermal stress Usually the residual stress pattern can be favorably changed by changing the deposition conditions b. Formation of brittle intermetallic compounds an ...
Chemistry - Pearson School
... This document demonstrates how Pearson Chemistry ©2012 meets the objectives of the New York Physical Setting/Chemistry Core Curriculum. Correlation page references are to the Student and Teacher’s Editions and are cited at the page level. Pearson Chemistry combines proven and tested content with cut ...
... This document demonstrates how Pearson Chemistry ©2012 meets the objectives of the New York Physical Setting/Chemistry Core Curriculum. Correlation page references are to the Student and Teacher’s Editions and are cited at the page level. Pearson Chemistry combines proven and tested content with cut ...
Time allotted: 3hours Maximum Marks: 70
... 19. Find out whether it is possible to reduce MgO using carbon at 298 K. If not at what temperature it become spontaneous. For reaction ...
... 19. Find out whether it is possible to reduce MgO using carbon at 298 K. If not at what temperature it become spontaneous. For reaction ...
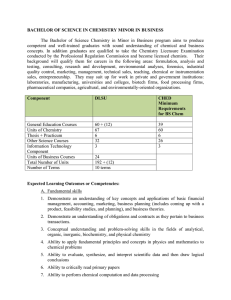
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... INOCHE2 General Chemistry 2 for Chemistry and Biochemistry Majors A continuation of General Chemistry 1 covering elementary chemical thermodynamics, chemical equilibrium, acidbase theories and applications, reduction-oxidation reactions, and kinetics. 3 units Pre-requisite: INOCHE1 INOCHE3 Inorgani ...
... INOCHE2 General Chemistry 2 for Chemistry and Biochemistry Majors A continuation of General Chemistry 1 covering elementary chemical thermodynamics, chemical equilibrium, acidbase theories and applications, reduction-oxidation reactions, and kinetics. 3 units Pre-requisite: INOCHE1 INOCHE3 Inorgani ...
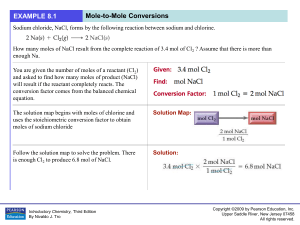
Stoichiometry: Predicting Amounts in Reactions
... Stoichiometry is the process of determining how much product is made or how much reactant is needed during a chemical reaction. As we know, in chemical reactions atoms are conserved. We show thi ...
... Stoichiometry is the process of determining how much product is made or how much reactant is needed during a chemical reaction. As we know, in chemical reactions atoms are conserved. We show thi ...