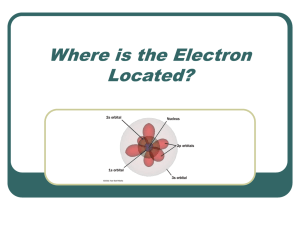
7.3-Flame Test Lab
... All metal elements have distinct properties. One of these properties is its own special color when burned in an open flame. This is caused by the flame exciting the electrons of the metal element in a compound. When the electrons return from the excited state to the ground state, a photon of energy ...
... All metal elements have distinct properties. One of these properties is its own special color when burned in an open flame. This is caused by the flame exciting the electrons of the metal element in a compound. When the electrons return from the excited state to the ground state, a photon of energy ...
AP Exam Two Retake Qualifying Assignment
... b. Electron configurations are only probable. c. Electron spins are more important than energy levels in determining electron configuration. d. Some elements have unusual atomic orbitals. How does the speed of visible light compare with the speed of X rays, when both speeds are measured in a vacuum? ...
... b. Electron configurations are only probable. c. Electron spins are more important than energy levels in determining electron configuration. d. Some elements have unusual atomic orbitals. How does the speed of visible light compare with the speed of X rays, when both speeds are measured in a vacuum? ...
Photoelectric Effect 1 Introduction 2 Experiment
... up to hf − W (most will have lost some additional energy moving through the metal). Of the ejected electrons, some will travel to the anode and so create a current between the two electrodes. By attaching a power supply to the electrodes and applying a voltage, V , which creates a repelling electric ...
... up to hf − W (most will have lost some additional energy moving through the metal). Of the ejected electrons, some will travel to the anode and so create a current between the two electrodes. By attaching a power supply to the electrodes and applying a voltage, V , which creates a repelling electric ...
Slide 1
... 35. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same ...
... 35. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same ...
Solution
... According to Lewis theory, what is wrong with this structure for hydrogen cyanide, HCN (mark all that apply)? ...
... According to Lewis theory, what is wrong with this structure for hydrogen cyanide, HCN (mark all that apply)? ...
constructive - Purdue Physics
... effect can be obtained with a prism, however a prism is a thick (triangular section) slab of glass and absorbs too much light for certain applications (for example star light). The spectrum of distant stars can be obtained by using a grating. It turns out that different elements have a special signa ...
... effect can be obtained with a prism, however a prism is a thick (triangular section) slab of glass and absorbs too much light for certain applications (for example star light). The spectrum of distant stars can be obtained by using a grating. It turns out that different elements have a special signa ...
Early Quantum Theory and Models of the Atom
... • Bohr studied Rutherford’s planetary model and found it had validity • But to make it work the newly developing quantum theory would have to be incorporated • Plank and Einstein had shown that in heated solids, the energy of oscillating electric charges must change from one discrete energy state to ...
... • Bohr studied Rutherford’s planetary model and found it had validity • But to make it work the newly developing quantum theory would have to be incorporated • Plank and Einstein had shown that in heated solids, the energy of oscillating electric charges must change from one discrete energy state to ...
Name - Red Hook Central Schools
... In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, φ. The kinetic energy of an ejected photo ...
... In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, φ. The kinetic energy of an ejected photo ...
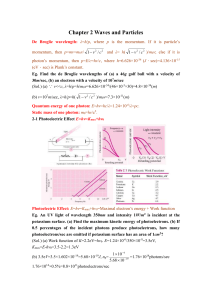
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
AP Chemistry
... Hydrogen bonding London dispersion forces Van der Waal forces Surface tension Capillary action Viscosity Crystalline solids Lattice unit cell Ionic solids Atomic solids (metallic, network, Group 18) Molecular solids Malleable Ductile Band model Alloys: substitutional, interstitial Diamonds vs graphi ...
... Hydrogen bonding London dispersion forces Van der Waal forces Surface tension Capillary action Viscosity Crystalline solids Lattice unit cell Ionic solids Atomic solids (metallic, network, Group 18) Molecular solids Malleable Ductile Band model Alloys: substitutional, interstitial Diamonds vs graphi ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... - We are assuming here that the electron is revolving around the fixed nucleus in a circular orbit of radius r. - Classically , because the electron is constantly being accelerated accoding to Eq. 1.21 , it should emit ...
... - We are assuming here that the electron is revolving around the fixed nucleus in a circular orbit of radius r. - Classically , because the electron is constantly being accelerated accoding to Eq. 1.21 , it should emit ...
Regents Review Packet B2 Answer Key
... elements are listed in the table below. A student's experimental result indicates that the density of element Q is , at room temperature and standard pressure. ...
... elements are listed in the table below. A student's experimental result indicates that the density of element Q is , at room temperature and standard pressure. ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.