3.3 Why do atoms radiate light?
... • Each state, which is not an Eigenstate of the Hamiltonian has a non infinite lifetime, which is defined in our example by the matrix element in Eq. (3.18). • The calculation of these matrix elements is the main task of quantum mechanics. • Non-zero matrix elements (even if the corresponding states ...
... • Each state, which is not an Eigenstate of the Hamiltonian has a non infinite lifetime, which is defined in our example by the matrix element in Eq. (3.18). • The calculation of these matrix elements is the main task of quantum mechanics. • Non-zero matrix elements (even if the corresponding states ...
Welcome to Physics 112N
... blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
... blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
Chapter 7 Name Atomic Structure and Periodicity Any day you don`t
... 2. For light with frequency lower than the threshold frequency, no electrons are emitted regardless of the intensity (amplitude) 3. For light with frequency greater than threshold frequency, the number of electrons emitted increases with intensity of light 4. For light with frequency greater than th ...
... 2. For light with frequency lower than the threshold frequency, no electrons are emitted regardless of the intensity (amplitude) 3. For light with frequency greater than threshold frequency, the number of electrons emitted increases with intensity of light 4. For light with frequency greater than th ...
Early Modern Physics
... • used Statistical Mechanics (we’ll do later in 461) to determine relative probability for any wavelength l • need::number of states (“nodes”) for any l - energy of any state probability versus energy • the number of states = number of standing waves = N(l)dl = 8pV/l4 dl with V = volume • Classical ...
... • used Statistical Mechanics (we’ll do later in 461) to determine relative probability for any wavelength l • need::number of states (“nodes”) for any l - energy of any state probability versus energy • the number of states = number of standing waves = N(l)dl = 8pV/l4 dl with V = volume • Classical ...
Chapter 6 * Electronic Structure of Atoms
... • Energy (light) is emitted or absorbed in discrete units (quantum) • Each metal has a different energy at when it emits electrons. At lower energy, electrons are not emitted. • Einstein used quanta to explain the photoelectric effect. Energy is proportional to frequency. E = h where h is Planck’s ...
... • Energy (light) is emitted or absorbed in discrete units (quantum) • Each metal has a different energy at when it emits electrons. At lower energy, electrons are not emitted. • Einstein used quanta to explain the photoelectric effect. Energy is proportional to frequency. E = h where h is Planck’s ...
The Spring 2006 Qualifying Exam, Part 1
... intensity. No photoelectrons are emitted for light with frequency lower than this, no matter how great the intensity of the light. (a) These observations cannot be explained consistently using classical physics. Describe how quantum mechanics, specifically that light may be assumed to come in quanti ...
... intensity. No photoelectrons are emitted for light with frequency lower than this, no matter how great the intensity of the light. (a) These observations cannot be explained consistently using classical physics. Describe how quantum mechanics, specifically that light may be assumed to come in quanti ...
Honors Chemistry
... 60. Xylitol is a sweetener that has anticavity properties because it does not stick to teeth. Elemental analysis of a sample resulted in 0.5921 g carbon, 0.1184 g hydrogen and 0.7895 g oxygen. The molar mass was determined by an effusion rate comparison with oxygen gas. Oxygen was found to effuse ...
... 60. Xylitol is a sweetener that has anticavity properties because it does not stick to teeth. Elemental analysis of a sample resulted in 0.5921 g carbon, 0.1184 g hydrogen and 0.7895 g oxygen. The molar mass was determined by an effusion rate comparison with oxygen gas. Oxygen was found to effuse ...
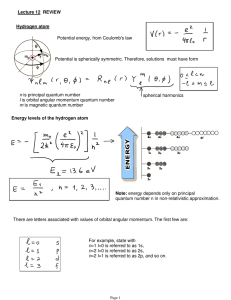
Lecture 12: Review.
... Hund's first rule: for every atomic ground state, the total electron spin has maximum value tolerated by the Pauli principle. Hund's second rule: for a given spin, the term with the largest value of the total orbital angular momentum quantum number L, consistent with overall antisymmetrization, has ...
... Hund's first rule: for every atomic ground state, the total electron spin has maximum value tolerated by the Pauli principle. Hund's second rule: for a given spin, the term with the largest value of the total orbital angular momentum quantum number L, consistent with overall antisymmetrization, has ...
Chapter 3 Make up Test 2004
... ______26. Which of the following statements explains why chemists do not count atoms and molecules directly? A. Atoms and molecules are extremely small B. All of the relationships in a chemical reaction can be expressed as mass ratios C. Matter is neither created nor destroyed in a chemical reaction ...
... ______26. Which of the following statements explains why chemists do not count atoms and molecules directly? A. Atoms and molecules are extremely small B. All of the relationships in a chemical reaction can be expressed as mass ratios C. Matter is neither created nor destroyed in a chemical reaction ...
Biol 1441
... Polar covalent bond: the electrons of the bond are not shared equally. Ex: HCl Ionic Bonds: Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ...
... Polar covalent bond: the electrons of the bond are not shared equally. Ex: HCl Ionic Bonds: Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ...
Chem20u2(5.2) - Mr. Searcy Chemistry 20
... Heisenberg uncertainty principle on the modern view of electrons in atoms. 5. Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic orbitals. II. The following questions will help to cover these objectives as you read through the section. Briefly answer each questio ...
... Heisenberg uncertainty principle on the modern view of electrons in atoms. 5. Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic orbitals. II. The following questions will help to cover these objectives as you read through the section. Briefly answer each questio ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.