No Slide Title - Rubin Gulaboski
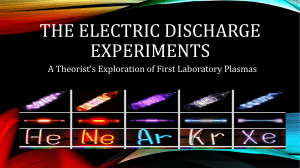
... Radiation composed of only one wavelength is called monochromatic. Most common radiation sources that produce radiation containing many different wavelengths components, a spectrum. This rainbow of colors, containing light of all wavelengths, is called a continuous spectrum. Note that there are no d ...
... Radiation composed of only one wavelength is called monochromatic. Most common radiation sources that produce radiation containing many different wavelengths components, a spectrum. This rainbow of colors, containing light of all wavelengths, is called a continuous spectrum. Note that there are no d ...
energy
... caused by an electron that had been excited away from their ground state near the nucleus, returning to its ground state. ...
... caused by an electron that had been excited away from their ground state near the nucleus, returning to its ground state. ...
File - Chemistry 11 Enriched
... Each element has a specific electron configuration defining where the electrons are located. In order to understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroad ...
... Each element has a specific electron configuration defining where the electrons are located. In order to understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroad ...
Semester Exam Review - Teach-n-Learn-Chem
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
Chapter 4: Arrangement of Electrons in Atoms
... 6. Bohr used this information to correctly predict the line spectrum of hydrogen. However, he could not correctly predict line spectra for other elements. Why? Interference from other electrons. II. Quantum Model of the Atom A. Electrons as Waves 1. In 1924 Louis de Broglie proposed that electrons ...
... 6. Bohr used this information to correctly predict the line spectrum of hydrogen. However, he could not correctly predict line spectra for other elements. Why? Interference from other electrons. II. Quantum Model of the Atom A. Electrons as Waves 1. In 1924 Louis de Broglie proposed that electrons ...
Chemistry I Honors – Semester Exam Review – Fall 2000
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m ...
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m ...
Fall Final Review Honors
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
Condensed Plasmoids – The Intermediate State of LENR
... The multi-electron system is approximated by computing a collection of one-electron orbitals, whereby each electron orbital is subjected to the mean electric potential and magnetic vector potential created by the total charge density and total current density of all other occupied orbitals and the n ...
... The multi-electron system is approximated by computing a collection of one-electron orbitals, whereby each electron orbital is subjected to the mean electric potential and magnetic vector potential created by the total charge density and total current density of all other occupied orbitals and the n ...
Chemistry for Bio 11
... • Molecules- 2 or more atoms combined in a specific way • Compounds- different elements in a molecule, in exact, whole-number ratios, joined by a chemical bond • 2 major means of intramolecular chemical bonding: Covalent (incl. polar and nonpolar) and Ionic ...
... • Molecules- 2 or more atoms combined in a specific way • Compounds- different elements in a molecule, in exact, whole-number ratios, joined by a chemical bond • 2 major means of intramolecular chemical bonding: Covalent (incl. polar and nonpolar) and Ionic ...
The Chemical Context of Life Chapter 2 Notes
... Atomic number: # of protons Mass number: sum of protons + neutrons Isotopes: different atomic forms of an element. -ex. Carbon-12 (99%), Carbon-13 (1%), Carbon-14 (<1%) ...
... Atomic number: # of protons Mass number: sum of protons + neutrons Isotopes: different atomic forms of an element. -ex. Carbon-12 (99%), Carbon-13 (1%), Carbon-14 (<1%) ...
Nano-material - McMaster University > ECE
... mixes all the quantized electron energy levels and the material shows a bulk behavior, i.e., the quantization feature is not preserved for the same type of electrons (with the same effective mass), but still preserved among different type of electrons, hence we have (discrete) energy bands If the co ...
... mixes all the quantized electron energy levels and the material shows a bulk behavior, i.e., the quantization feature is not preserved for the same type of electrons (with the same effective mass), but still preserved among different type of electrons, hence we have (discrete) energy bands If the co ...
Unit 16 Worksheet - Jensen Chemistry
... Review: 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before ...
... Review: 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before ...
Solutions Fall 2004 Due 5:01 PM, Tuesday 2004/10/12
... Solution: Crystallographic studies rely on the interference effects of the incident beam when reflected or refracted off of the various planes making up the crystal lattice being studied. This type of interference depends on the wavelength of the beam, and the interaction between the beam constituen ...
... Solution: Crystallographic studies rely on the interference effects of the incident beam when reflected or refracted off of the various planes making up the crystal lattice being studied. This type of interference depends on the wavelength of the beam, and the interaction between the beam constituen ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.