Microsoft Word
... Resonance Structures Some molecules may have two or more equivalent Lewis structures. These are known as resonance structures. This does not mean that the molecule jumps back and forth between these structures. In reality, the true structure of the molecule will lie somewhere in between these reson ...
... Resonance Structures Some molecules may have two or more equivalent Lewis structures. These are known as resonance structures. This does not mean that the molecule jumps back and forth between these structures. In reality, the true structure of the molecule will lie somewhere in between these reson ...
PHOTOEMISSION SPECTROSCOPY-Duino 2009
... interaction of electron with matter imposes to perform experiments under vacuum (at least 107 mbar) in order to measure a probability distributions of photoelectrons (Je(hν,Ee,θ,φ,σ)) not altered by interaction with the background atmosphere surrounding the sample. As far as photon source is concern ...
... interaction of electron with matter imposes to perform experiments under vacuum (at least 107 mbar) in order to measure a probability distributions of photoelectrons (Je(hν,Ee,θ,φ,σ)) not altered by interaction with the background atmosphere surrounding the sample. As far as photon source is concern ...
Chapter 10 Notes
... what happens when hydrogen gas in a tube is heated to high temperatures or excited by passing an electric current through it. ...
... what happens when hydrogen gas in a tube is heated to high temperatures or excited by passing an electric current through it. ...
Document
... Molarity, or moles per liter (M) A mole of an element or compound is equal to its atomic or molecular weight (sum of atomic weights) in grams One mole of any substance contains exactly the same number of solute particles (6.02 x 1023) 37. Colloids and Suspensions Colloids, or emulsions, are heteroge ...
... Molarity, or moles per liter (M) A mole of an element or compound is equal to its atomic or molecular weight (sum of atomic weights) in grams One mole of any substance contains exactly the same number of solute particles (6.02 x 1023) 37. Colloids and Suspensions Colloids, or emulsions, are heteroge ...
HW / Unit 2
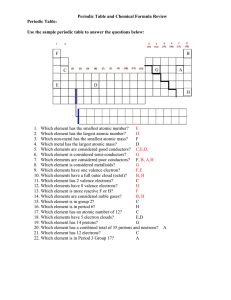
... 6. Place the following atoms in order of increasing size: S, Rb, K, C, O, Al, P 7. What happens to the size of an atom when it loses an electron? Gains an electron? 8. Place the following atoms and ions in order of increasing size: Cl, Cl-, Mg, Mg2+ 9. Which element is the most common in the univers ...
... 6. Place the following atoms in order of increasing size: S, Rb, K, C, O, Al, P 7. What happens to the size of an atom when it loses an electron? Gains an electron? 8. Place the following atoms and ions in order of increasing size: Cl, Cl-, Mg, Mg2+ 9. Which element is the most common in the univers ...
Lecture 1 Atomic Structure
... • Nodal plane – the plane cutting through a nucleus, separating the region of + and – sign of the wavefunction. (Note the bright and dark lope which is opposite to each other.) • No electrons at nodal plane • There are three ml values (-1, 0, and +1) for p-orbitals, representing px, py and pz. I wil ...
... • Nodal plane – the plane cutting through a nucleus, separating the region of + and – sign of the wavefunction. (Note the bright and dark lope which is opposite to each other.) • No electrons at nodal plane • There are three ml values (-1, 0, and +1) for p-orbitals, representing px, py and pz. I wil ...
Photoelectric Effect
... respectively. If both metals are illuminated by white light (wavelengths between 400nm and 700nm), which one gives off photoelectrons with the greater maximum kinetic energy? Assuming electrons are ejected from both metals, the answer should be cadmium, because it has a lower work function – less en ...
... respectively. If both metals are illuminated by white light (wavelengths between 400nm and 700nm), which one gives off photoelectrons with the greater maximum kinetic energy? Assuming electrons are ejected from both metals, the answer should be cadmium, because it has a lower work function – less en ...
AP Chemistry Second Semester Notes
... 1. uncertainty in measurements a. Dalton's atomic theory (1805) a. data analysis 1. unique, indestructible atoms for each element 1. accuracy = correct (even if inconsistent) is 2. atoms are rearranging, not created during measured by percent difference: chemical change % = 100|mean – true|/true 3 ...
... 1. uncertainty in measurements a. Dalton's atomic theory (1805) a. data analysis 1. unique, indestructible atoms for each element 1. accuracy = correct (even if inconsistent) is 2. atoms are rearranging, not created during measured by percent difference: chemical change % = 100|mean – true|/true 3 ...
Exam #2
... respectively, which organism could get more energy for growth, organism A that oxidizes methane and reduces sulfate, or organism B that oxidizes hydrogen sulfide and reduces iron? Justify your answer in terms of volts. It may be useful to diagram the electron transfer between couples for each. Organ ...
... respectively, which organism could get more energy for growth, organism A that oxidizes methane and reduces sulfate, or organism B that oxidizes hydrogen sulfide and reduces iron? Justify your answer in terms of volts. It may be useful to diagram the electron transfer between couples for each. Organ ...
General Chemistry
... Group 7 (halogens) have the most negative electron affinity because it have a room in it valence shell for an additional electron, Group 2 and Nobel gases have electron affinity near zero or positive, the above figure show how the Ionization energy increase through groups and periods. Electron affin ...
... Group 7 (halogens) have the most negative electron affinity because it have a room in it valence shell for an additional electron, Group 2 and Nobel gases have electron affinity near zero or positive, the above figure show how the Ionization energy increase through groups and periods. Electron affin ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.